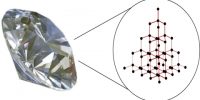
Sol-Gel method: A sol is a colloidal dispersion of small (1-1000 nm diameter) articles. In the Sol-Gel method a sol of reactants in a suitable solvent is first prepared. The sol is then either treated or simply left to form gel. The gel is finally heated to obtain the desired product. The main steps in Sol-Gel method is schematically shown in Figure.
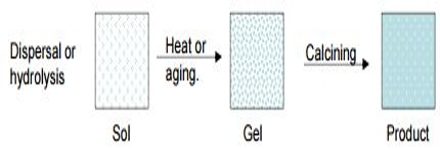
Fig: Schematic diagram of sol-gel method
The heating of the gel helps so remove the solvent and allows the rearrangement in the structure of the solid. It also allows crystallization.
Sol-Gel chemistry means Polymerize a solution of precursor molecules to form a sol or gel.
Si(OEt)4(soln) + 2H2O → SiO2 (gel) + 4EtOH
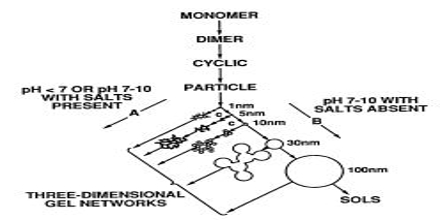
Steps in sol formation
Precursor initially undergoes hydrolysis
– Si(OEt)4 + H2O → Si(OEt)3 OH + EtOH
Then get condensation
– Si(OEt)3 OH + Si(OEt)3 OH → (EtO) 3SiOSi(OEt)3 + H2O
– Or Si(OEt)3 OH + Si(OEt)4 → (EtO)3 SiOSi(OEt)3 + EtOH
Both steps are often accelerated using an acid or base catalyst
Advantages sol-gel chemistry
- Cheap way to make thin films and fibres compared to CVD but can be expensive relative to heat and beat
- Molecular mixing of precursors prior to hydrolysis helps get good homogeneity
- Can make high purity materials
- Low firing temperatures