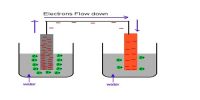
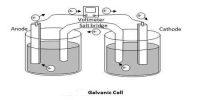
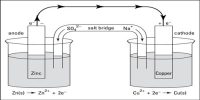
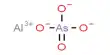
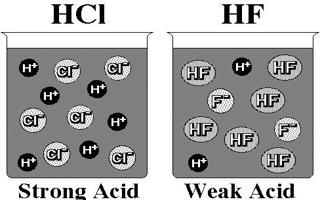
We know that in a solution of a strong acid one can normally ignore the self-ionisation of water as a source of a few extra H+ (aq). The concentration of H+ ions is usually determined just from the strong acid concentration. However, the self-ionisation still exists so there is a small concentration of OH- ions. Similarly, in a solution of a strong base, the self- ionisation still exists and is responsible for a small concentration of H+ ions, while the concentration of OH- ions is usually determined by the strong base concentration.
Example
Calculate the concentration of H+ ions in 0.010 M NaOH.
NaOH is a strong base then [OH-] = 0.010 M. Substituting [OH-] = 0.010 M into the ion-product expression, we get:
1.0 x 10-14 = (0.010) x [H+]
[H+] = 1.0 x 10-12 M