Interhalogen compounds are generally covalent compounds in which the larger halogen forms the central atom.
- Type AX. As excepted, the compounds of the type AX are linear. Thus CΙF, BrF, BrCl, ΙBr and ΙCI are all linear in structure.
Electronic structure of Chlorine atom, in the ground state and hybridized state is represented as in Figure.
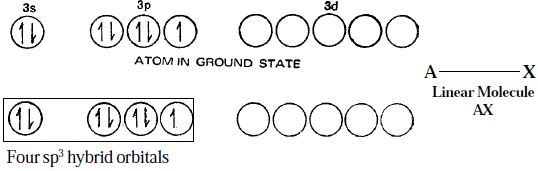
Figure: Linear structure of the interhalogen compounds of the type AX
Although the spatial arrangement of the four electron pairs (bp = 1 and lps = 3) round the central chlorine atom is tetrahedral, due to the presence of three lone pairs of electrons in three hybrid orbitals, the shape of AX molecule gets distorted and become linear.
- Type AX3 Compounds of the type AX3 have trigonal bipyramidal structure, Figure a, for the ClF3 molecule.
Bipyramidal structure arises out of sp3d hybridization involved in the formation of this compound, as illustrated in the Figure b. The three dotted arrows indicate electrons contributed by the three fluorine atoms (without lone pair it is T-shaped).
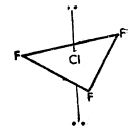
Fig.a: Bi pyramidal structure of CIF3 molecule
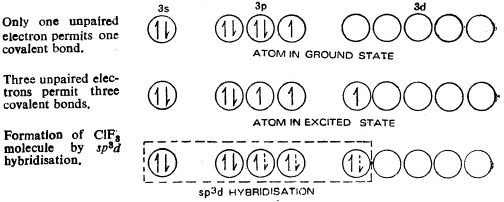
Fig.b: sp3d hybridization involved in the formation of ClF3 molecule