Disordered yet Highly Symmetrical Structure: Making disorder for a perfect battery
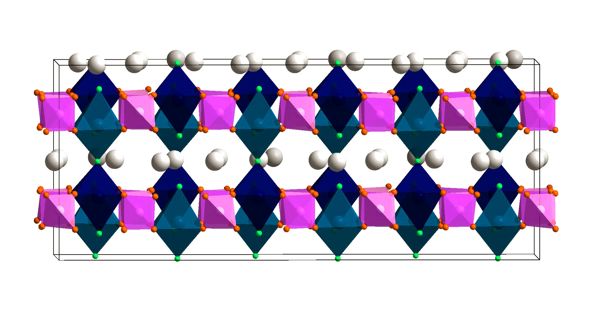
Manufacturing safer, more powerful batteries that use geopolitically stable resources requires solid electrolytes and replacing lithium with sodium. The lithium batteries that power our electronic devices and electric vehicles have a number of drawbacks. It’s a winning combination that also means it is possible to manufacture batteries that are more powerful. The electrolyte is a flammable liquid and the lithium they’re made of is a limited resource. The properties of these “ideal” batteries would be based on the crystalline structure of the electrolyte, a hydroborate consisting of boron and hydrogen. Specialists have now developed a non-flammable, solid electrolyte that operates at room temperature. It transports sodium – which is found everywhere on earth – instead of lithium.
Specialists in crystallography at the University of Geneva (UNIGE) have developed a non-flammable, solid electrolyte that operates at room temperature.
The lithium batteries that power our electronic devices and electric vehicles have a number of drawbacks. The electrolyte — the medium that enables electrons and positive charges to move between the electrodes — is a flammable liquid. What’s more, the lithium they’re made of is a limited resource that is the focus of major geopolitical issues. Specialists in crystallography at the University of Geneva (UNIGE) have developed a non-flammable, solid electrolyte that operates at room temperature. Indeed, the development of electric vehicles that do not emit greenhouse gases hinges on the existence of powerful, safe batteries, just as the development of renewable energies – solar and wind – depends on energy storage capacities.
It transports sodium — which is found everywhere on earth — instead of lithium. It’s a winning combination that also means it is possible to manufacture batteries that are more powerful. The properties of these “ideal” batteries would be based on the crystalline structure of the electrolyte, a hydroborate consisting of boron and hydrogen.
The obstacle of storing electricity is colossal for sustainability initiatives. Without a doubt, the advancement of electrical autos that do not emit greenhouse gases hinges on the existence of impressive, harmless batteries, just as the advancement of renewable energies — photovoltaic and wind — depends on electricity storage capacities. Lithium batteries are the recent reply to these worries. Regrettably, lithium demands liquid electrolytes, which are remarkably explosive in the event of a leak. “What is actually a lot more, lithium isn’t really found everywhere on earth, and it results in geopolitical concerns very similar to all those encompassing oils. Sodium is a very good prospect to switch it because it has chemical and bodily homes near to lithium and is found everywhere,” argues Fabrizio Murgia, an article-doctoral fellow in UNIGE’s School of Sciences.
Too high a temperature
The two features — sodium and lithium — are around every single other in the Periodic Table. “The problem is that sodium is heavier than its cousin lithium. That implies it has an issue generating its way close to in the battery electrolyte,” adds Matteo Brighi, an article-doctoral fellow at UNIGE and the study’s initial writer. Accordingly, there is a will need to produce electrolytes capable of transporting cations such as sodium. In 2013 and 2014, Japanese and American analysis teams recognized hydroborates as very good sodium conductors at about 120°C. At initially look, this is an excessive temperature for everyday use of batteries… but a godsend for the Geneva laboratory!
With decades of abilities in hydroborates employed in applications such as hydrogen storage, the Geneva crystallographers established doing the job of decreasing the conduction temperature. “We obtained extremely very good benefits with outstanding homes suitable for batteries. We succeeded in utilizing hydroborates as an electrolyte from space temperature to 250 degrees Celsius with no basic safety concerns. What is actually a lot more, they resist increased possible dissimilarities, that means the batteries can retailer a lot more electricity,” proceeds Radovan Cerny, a professor in UNIGE’s Laboratory of Crystallography and challenge chief.