Allergic disorders such as anaphylaxis, hay fever, eczema, and asthma now affect approximately 25% of the population in the developed world. Chronic allergic inflammation results from repeated or persistent exposure to allergens, which are typically innocuous substances found in the environment. This, in turn, causes long-term structural changes in the affected organs as well as significant functional abnormalities. As a result, it is critical to comprehend the characteristics and consequences of acute and chronic allergic inflammation, as well as to investigate how mast cells can contribute to several aspects of this maladaptive pattern of immunological reactivity.
A new study reveals new information about the body’s type 2 innate immune response system. New findings about a built-in rapid reaction system that triggers inflammatory responses when people are exposed to allergens like insects, mites, and fungi may also hold the key to helping more people manage their allergies in the coming years.
A study led by Cincinnati Children’s researchers and published in Nature Immunology on September 16, 2021, reveals new information about the body’s “type 2 innate immune response” system. The discovery of a common biological response platform suggests that any new medication that can control the response could benefit people with a wide range of allergies.
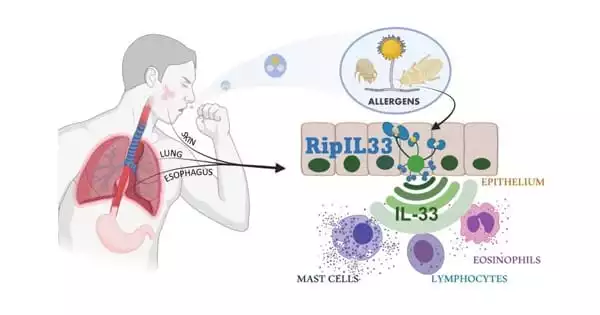
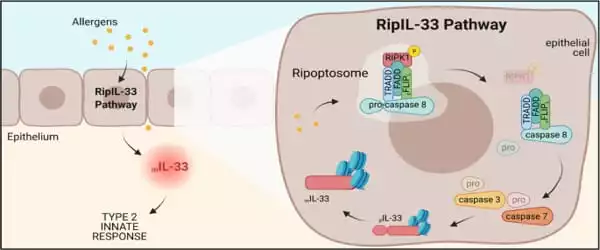
New insights into the role of ripoptosome signaling and caspases in allergic inflammation enabled this breakthrough. We discovered that ripoptosome activation markers and mature IL-33 levels dynamically correlated with the degree of esophageal eosinophilia and disease activity in the human allergic disease eosinophilic esophagitis (EoE).
Michael Brusilovsky
“Disrupting this allergen sensing pathway could provide a unique opportunity to counteract type 2 immunity and alleviate allergic inflammation,” says Marc Rothenberg, MD, Ph.D., Director of the Division of Allergy and Immunology at Cincinnati Children’s and the study’s senior author.
The research team included Michael Brusilovsky, MMedSc, Ph.D., Mark Rochman, Ph.D., Yrina Rochman, Ph.D., Julie Caldwell, Ph.D., Lydia Mack, MS, Jennifer Felton, Ph.D., Jeff Habel, Ph.D., Aleksey Porollo, Ph.D., and Chandrashekhar Pasare, DVM, Ph.D., in addition to Rothenberg.
Previous research had shown that when allergens penetrate the epithelial layer of mucosal membranes, they cause a similar IL-33 response. The mechanisms at work in the process were identified by the Cincinnati Children’s team.
Allergen sensing system
“New insights into the role of ripoptosome signaling and caspases in allergic inflammation enabled this breakthrough,” says Brusilovsky, the study’s first author. The allergens, in particular, activate an interconnected set of cell death-inducing signals known as the ripoptosome. This signaling “platform” contains numerous components, but the key player in allergic inflammatory reactions appears to be a molecular switch called caspase 8. The pathway was named “RipIL-33” by the researchers because IL-33 is processed (ripped) by the ripoptosome.
Immunologists have discovered the mechanisms by which bacteria and viruses are sensed by the innate immune system in the last two decades, but how allergens are sensed has remained a mystery.
“The discovery of this unexpected mechanism is the most significant breakthrough in understanding how the innate immune system senses allergens to initiate a type 2 response and subsequent allergic inflammation,” says Pasare, one of the study’s senior authors.
Caspase 8 inhibition reduced the IL-33 response to allergen exposure in mice and limited bronchial inflammation in the lungs. Further research revealed that a similar process occurs in humans.
“We discovered that ripoptosome activation markers and mature IL-33 levels dynamically correlated with the degree of esophageal eosinophilia and disease activity in the human allergic disease eosinophilic esophagitis (EoE),” the study says.
Nature Immunology editors chose this paper to be featured on the cover of the September print edition. The next step is to confirm the RipIL-33 pathway in human allergic reactions and see if existing drugs or a new compound can safely disrupt the inflammation cycle.