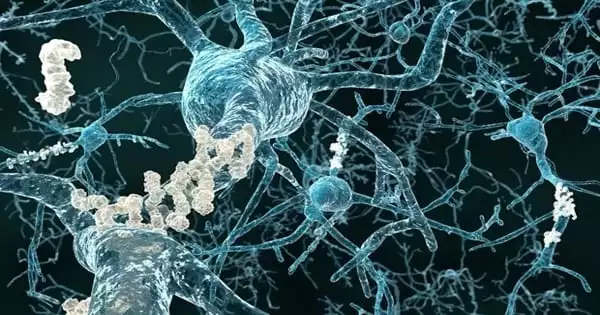
Researchers discovered that gene variants linked to an increased risk of developing Alzheimer’s disease disrupt the brain’s natural protective mechanism against the disease. The brain has a natural protective mechanism against Alzheimer’s disease, and researchers at Baylor College of Medicine, Texas Children’s Hospital, and other institutions have discovered that gene variants linked to the disease’s risk disrupt the protective mechanism in ways that can lead to neurodegeneration. In a fruit fly model of the condition, the researchers also demonstrated that an ABCA1 agonist can restore certain alterations in the brain’s protective mechanism.
The researchers present evidence that reactive oxygen species (ROS), natural byproducts of cellular metabolism linked to inflammation and other processes, are key players in the events that lead to the disruption of the neuroprotective mechanism. Furthermore, the researchers discovered that the combination of ROS and amyloid-beta, the main component of plaques found in the brains of people with Alzheimer’s disease, accelerated disease development in animal models. Overall, the findings provide new mechanistic insight into factors involved in Alzheimer’s disease development, lending support to the idea that multiple genetic and other cellular changes combine to cause the disease. The findings were published in the Proceedings of the National Academy of Sciences.
“Previous research by Dr. Lucy Liu in Dr. Hugo Bellen’s lab and colleagues demonstrated that two types of brain cells, neurons, and glia, work together to protect against neurodegeneration,” said first author Dr. Matthew Moulton, a postdoctoral associate in the Bellen lab. “In the current study, we used fruit fly and mammalian models to see if known genetic risk factors for Alzheimer’s disease were associated with disrupting the protective mechanism, delving into the details of how this happened.”
We wanted to identify genes that are critical for lipid droplet formation in the current study, specifically genes that are required for lipid export from neurons and lipid import into glia.
Dr. Lucy Liu
When neurons are exposed to high levels of ROS, the neuroprotective mechanism kicks in, causing neurons to produce an abundance of lipids. ROS levels rise with age, as a result of various forms of stress, or due to genetic factors. The reaction of ROS and lipids results in peroxidated lipids, which harm cellular health. Neurons try to avoid damage by secreting these lipids, which are then carried to glia cells by apolipoproteins, proteins that transport lipids. Glia sequester lipids in lipid droplets, keeping them out of the environment and away from neurons.
Previously, the researchers linked the neuroprotective mechanism to apolipoprotein APOE4, the strongest genetic risk factor for Alzheimer’s disease. “We discovered that APOE4 is virtually incapable of transferring lipids to glia, whereas the other two forms of APOE, APOE2 and APOE3, do so effectively,” said Bellen, Distinguished Service Professor of molecular and human genetics at Baylor. “When APOE4 is present, lipid droplet accumulation in glia is greatly reduced, and the protective mechanism fails. This fundamental difference in function in APOE4 likely predisposes an individual to the damaging effects of ROS, which increase with age.”
“We wanted to identify genes that are critical for lipid droplet formation in the current study, specifically genes that are required for lipid export from neurons and lipid import into glia,” Moulton explained. “We looked at genes that interact with APOE in neurons to get the lipids out, as well as genes that interact with APOE in glia to get the lipids in. One reason we’re interested in this is that human studies have shown that genes involved in both lipid import and export have been linked to Alzheimer’s disease and other conditions.”
One gene at a time, the researchers investigated the role of these Alzheimer’s risk genes in a fruit fly model. The model enabled them to see the effect of knocking down a specific gene, either in neurons or glia, on the formation of lipid droplets as well as neurodegeneration in the presence or absence of ROS.
“In all cases where ROS was present and we saw droplet loss, we also saw neurodegeneration, confirming that perturbations in glia droplet formation can lead to neuronal damage,” Moulton explained.
Using this method, the researchers demonstrated that several genes associated with the risk of developing Alzheimer’s disease in genome-wide sequencing studies disrupted neuroprotective lipid droplet formation, providing a mechanism to explain the risk associated with these genes.
Furthermore, Moulton and his colleagues used the fruit fly model to see if an ABCA1 agonist, which had previously been shown to restore APOE4’s ability to transfer lipids, could enable APOE4 to mediate lipid droplet formation in glia in the fruit fly model. “The ABCA1 agonist restored glial lipid droplet formation in an APOE4 fruit fly model, highlighting a potentially therapeutic avenue to prevent ROS-induced neurotoxicity,” said Bellen, Chair in Neurogenetics at Texas Children’s Hospital.
The researchers also looked into whether ROS could exacerbate the effects of amyloid-beta on the disease. “We found that combining ROS and amyloid-beta increased neuronal death in fruit flies and resulted in larger and more numerous amyloid-beta-rich plaques in a mouse model, indicating that ROS and amyloid-beta can interact and potentially influence disease progression,” Moulton explained.
“ROS levels in the brain rise as we age. If there are also mutations that disrupt the droplet pathways, neurons can become sensitive to the accumulation of lipid droplets, which can lead to neurodegeneration “Bellen stated. “Our findings support further research into feasible methods of reducing ROS levels in the brain as a strategy to minimize ROS’s key contribution to neurodegeneration.”