According to a study from Weill Cornell Medicine and Cornell’s Ithaca campus, aggressive and relatively common lymphomas known as diffuse large B cell lymphomas (DLBCLs) have a critical metabolic vulnerability that can be exploited to trick these cancers into starving themselves.
The study, published in Blood Cancer Discovery, found that a protein called ATF4, a genetic master switch that controls the activities of hundreds of genes, plays an important role in promoting the rapid growth of DLBCLs. The researchers discovered that silencing ATF4 in DLBCL cells effectively fools the cells into starving themselves and slowing their growth – and that targeting ATF4 in conjunction with a closely related metabolic protein, SIRT3, enhances this cancer-killing effect even further.
“ATF4 represents a critical and exploitable vulnerability in DLBCLs – and one that they appear to share regardless of the specific genetic mutations that trigger them,” said study co-senior author Dr. Ari Melnick, the Gebroe Family Professor of Hematology / Oncology in the Division of Hematology and Clinical Oncology and a member of the Sandra and Edward Meyer Cancer Center at Weill Cornell Medicine.
Dr. Hening Lin, a professor in the Department of Chemistry and Chemical Biology at Cornell University in Ithaca and a Howard Hughes Medical Institute investigator, is the study’s other co-senior author.
One of the really interesting things about this study is that it shows how nutrient conditions, in principle even from patients’ diets, can profoundly affect cancer-cell activity. ATF4 represents a critical and exploitable vulnerability in DLBCLs and one that they appear to share regardless of the specific genetic mutations that trigger them.
Dr. Ari Melnick
Lymphomas are blood cancers that typically develop from immune cells such as B cells, which produce antibodies. The vast majority of lymphomas are non-Hodgkin lymphomas, with DLBCLs accounting for roughly one-third of these, or approximately 25,000 cases per year in the United States. DLBCLs are relatively fast-growing and aggressive, and despite significant advances in lymphoma treatment in recent decades, approximately 40% of cases are not cured, highlighting the need for new treatment strategies.
Dr. Melnick, Dr. Lin, and their colleagues conducted the study to look into SIRT3, which is found in mitochondria, which are tiny, oxygen-burning fuel reactors in our cells that are essential for powering cellular activities. In a 2019 study, the researchers discovered that SIRT3 strongly supports the growth and survival of DLBCLs by speeding up the biochemical reactions that produce the molecular building blocks cells require to proliferate.
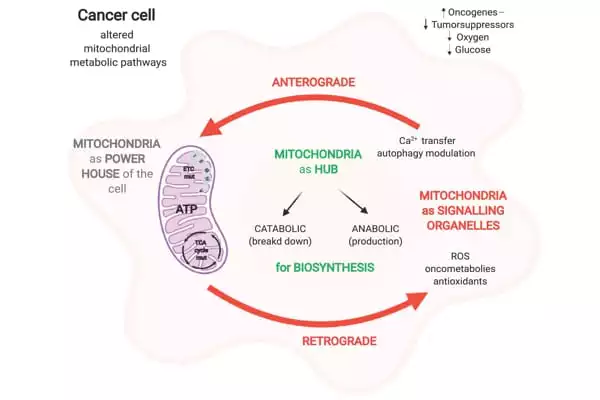
The new study looked into how SIRT3 promotes DLBCL growth further and discovered that one of the important ways it does so is by increasing the production of another metabolism-influencing protein, ATF4.
Their findings show that SIRT3, by boosting DLBCL metabolism, decreases the pools of amino acids that cells use to make proteins and otherwise fuel their growth. This reduction acts as a starvation signal, activating the production of ATF4, which in turn increases the production and import of amino acids, sustaining the malignant proliferation of DLBCLs.
In their 2019 study, Dr. Melnick and Dr. Lin created a selective SIRT3 inhibitor and demonstrated that it kills DLBCL cells regardless of the cancer-causing mutations they carry. The new study found that inhibiting SIRT3 causes an accumulation of specific amino acids, which are produced by the treated cells cannibalizing their own proteins. This situation essentially tricks DLBCL cells into acting as if they have adequate nutrient supplies, resulting in paradoxical suppression of ATF4 production, which leads to more severe starvation.
To further exploit this effect for therapeutic benefit, the researchers tested a compound that inhibits ATF4 activation and discovered that it has a similar broad impact on DLBCL cells. Furthermore, they discovered that combining the ATF4 and SIRT3 blockers has a much stronger lymphoma cell-killing effect than either blocker alone. Combining ATF4 and SIRT3 inhibitors appears to be a promising strategy for treating DLBCLs.
“One of the really interesting things about this study is that it shows how nutrient conditions, in principle even from patients’ diets, can profoundly affect cancer-cell activity,” said study first author Dr. Meng Li, a member of the Melnick Laboratory and a Weill Cornell Medicine instructor of cancer genomics. The team is now conducting further experiments to find the best way to target the SIRT3-ATF4 axis to treat DLBCLs.
“Despite the fact that my lab has been working on the sirtuin family of enzymes for over ten years, this study revealed some very interesting connections between SIRT3, metabolism, and nutrient or stress sensing,” Dr. Lin said. “We are very excited to think about the translational potential of this discovery in treating lymphoma.”
Dr. Ari Melnick has provided consulting services to Epizyme, Constellation, and Jubilant. Dr. Melnick serves on the KDAC Pharma advisory board.