This has in some degree been anticipated in the previous section. Vapour pressures of pure methane and pure propane are of course, at a particular temperature, fixed and invariant. In practice LNG will contain a few chemical species other than methane including higher hydrocarbons and diluent nitrogen. LPG might well be a blend of propane and butane, possibly with the latter in preponderance in which case the vapour pressure will be greatly reduced. LNG and LPG are both good fuels for spark ignition engines, although the former has not yet come into such use widely. Their vapour pressures are very much higher than the Reid Vapour Pressures given for gasolines in an earlier chapter. This is relevant to utilisation and to the storage and transportation which precede it.
Comparison of properties
The table below gives properties of liquid methane and of liquid propane. Comments, by way of comparison with other liquid fuels considered in this volume, will be made.
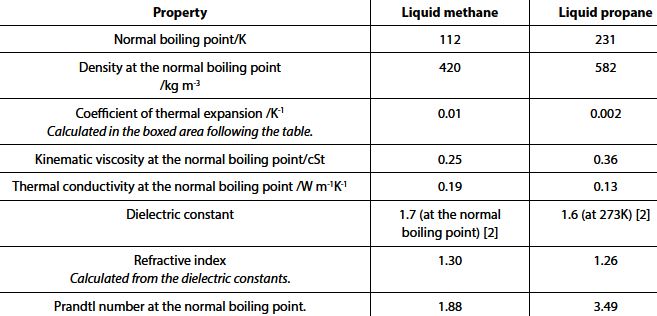
The kinematic viscosities in the fourth row of the table conform fairly closely to those given at room temperature (that is, at temperatures much higher than the normal boiling point either of methane or of propane) in Chapter 1 for a number of pure organic compounds. The value for liquid propane at 292 K is 0.22 cSt which must be seen as the value for LPG composed primarily of propane or with propane in a very high preponderance.













