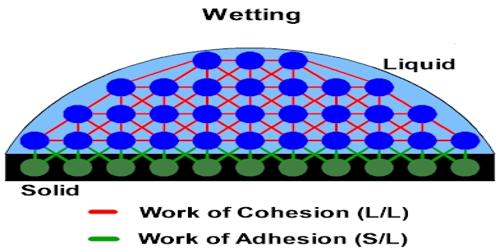
Adhesive Force: If a material is kept in touch with another material then there exists a mutual attractive force between the molecules of the two materials. Force of attraction among the molecules of different materials is called adhesive force. If water is kept in a container, then an attractive force acts between the molecules of water and the container which is the adhesive force. They are caused by forces acting between two substances, such as mechanical forces (sticking together) and electrostatic forces (attraction due to opposing charges). They are caused by forces acting between two substances, such as mechanical forces (sticking together) and electrostatic forces (attraction due to opposing charges). The force of adhesion is defined as the force of attraction between different substances, such as glass and water.
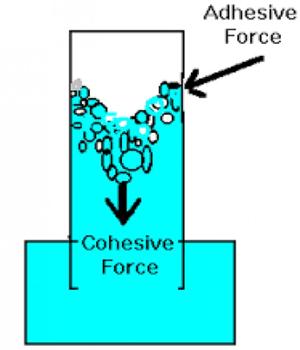
Adhesion is responsible for a meniscus when water is observed in a glass container because the water clings to the glass around the edges. Cohesion is responsible for surface tension, such as droplets of water beading together on waxed paper. Adhesive forces are attractive forces between molecules of different materials. Example- water molecule and silica