The carbohydrate given in the stimulus is glucose (C6 H12 O6). Structure is as follows:
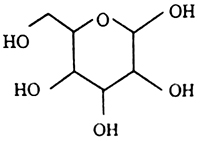
It is observed that, there is present a lots of hydroxyl (- OH) groups at glucose molecule. The molecules of glucose are connected by an intermolecular force with the water molecules and the mechanism of their connection is known as “hydrogen bond”.
When a hydrogen atom is covalently bonded to a highly electronegative atom, then the electron pair forming the bond is very much shifted to that atom and it acquires a partial negative charge and the hydrogen atom a partial positive charge. Since there are no more electrons in the hydrogen atom, its nucleus becomes partially dishielded from the electron cloud. In such polar compound the hydrogen atom is attracted strongly by neighboring electronegative atom of another molecule. This attraction is known as hydrogen bond.
Thus, the molecules of water and the molecules of carbohydrate arc connected with each other by hydrogen bond.
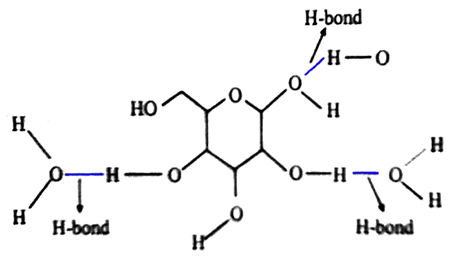
Hydrogen bond in Honey