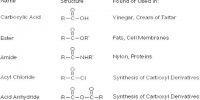
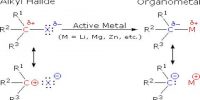Diasteriomer: If two optically active chiral compounds don’t act as mirror images of each other, they are named as `diasteriomers’ of each other. Diastereomers can have different physical properties and reactivity. They have different melting points and boiling points and different densities. They have two or more stereocenters.
Example: the two isomers of 3-chlorobutanol.
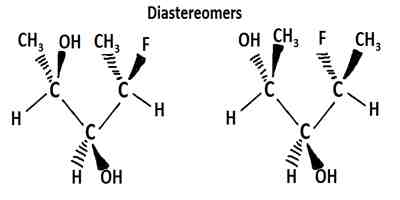
They rotate the plane of plane-polarized light towards same direction but at different degree and the mirror images are super-imposable on each other.