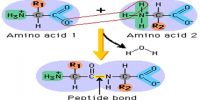
Electron affinity or electron gain enthalpy is the amount of energy released when an isolated gaseous atom accepts an electron to form a monovalent gaseous anion.
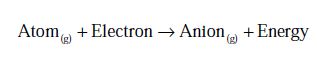
Electron gain enthalpies generally increase on moving from left to right in a period. Electron gain enthalpies generally decrease on moving down the group.