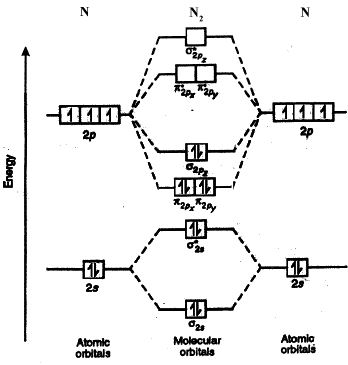
Nitrogen molecule (N2) is the electronic configuration of nitrogen (Z=7) in the ground state is 1s22s22px12py12pz1. Therefore, the total number of electrons present in the nitrogen molecule (N2) is 14. These 14 electrons can be accommodated in the various molecular orbitals in order of increasing energy.
N2 : KK (σ1s)2 (σ2s)2 (π2px)2 (π2py)2 (π2pz)2
Here, (σ1s)2 (σ2s)2 part of the configuration is abbreviated as KK, which denotes the K shells of the two atoms. In calculating bond order, we can ignore KK, as it includes two bonding and two antibonding electrons.
The molecular orbital energy level diagram of N2 is given in Figure.