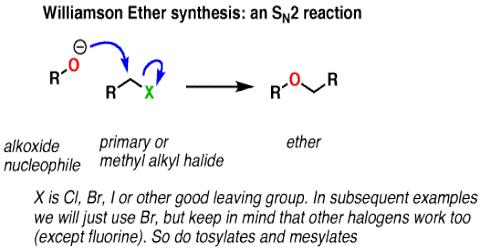
Williamson Ether Synthesis:
If alkyl halide halide is heated with the alcoholic (ethanol) solution of sodium alcoxide e.g. ethoxide (derived from ethanol & Na); ether is produced. It is an organic reaction, forming an ether from an organohalide and a deprotonated alcohol (alkoxide). This process of ether synthesis is known as Williamson Ether Synthesis.
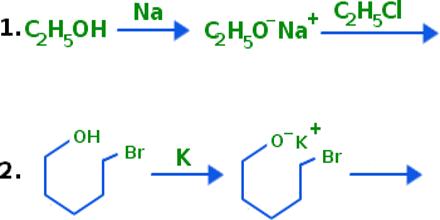
Example: The Williamson ether synthesis is a reaction that converts alcohols (R-OH) into ethers (R-O-R). The first step in this reaction is forming the conjugate base of the alcohol (called an alcoxide) by reacting the alcohol with sodium metal. This reaction forms hydrogen gas (H2) as a biproduct, so if you perform this reaction take caution to keep all flame sources away during sodium addition.
If ethyl bromide & sodium ethoxide mixture is heated with ether solution, ethoxy ethane or di-ethyl ether is produced.
H3CCH2 (Br+Na)O CH2CH3 → dry ether→ H3CCH2 – O – CH2CH3 + NaBr
Instead of alcoxide, alcohol can be used in this reaction, but the rate of reaction with alcohol is slower than that with alcoxide.