Only supportive and symptomatic therapy are available for the vast majority of people with mitochondrial disorders. However, novel medicines are being created in the laboratory and are entering human clinical trials as a result of amazing progress in understanding the causes and pathomechanisms of these various ailments over the previous decade.
Defective mitochondria – the ‘batteries’ that power our bodies’ cells – could be corrected in the future via gene-editing techniques. Scientists at the University of Cambridge have demonstrated that the mitochondrial DNA can be modified in live mice, paving the way for new treatments for incurable mitochondrial illnesses.
Mitochondrial illness, also known as mitochondrial dysfunction, refers to a range of conditions that affect the mitochondria, which are small compartments found in practically every cell of the body. The primary purpose of the mitochondria is to generate energy. More mitochondria are required to produce more energy, particularly in high-energy-demanding organs such as the heart, muscles, and brain. When the number or function of mitochondria in a cell is disrupted, less energy is produced, and organ failure occurs.
Our cells contain mitochondria, which provide energy for our cells to function. A tiny bit of mitochondrial DNA codes for each of these mitochondria. Mitochondrial DNA accounts for barely 0.1 percent of the total human genome and is passed down exclusively from mother to child.
Our research clearly has a long way to go before it can lead to a cure for mitochondrial illnesses. However, it demonstrates the possibility of a future treatment that eliminates the intricacy of mitochondrial replacement therapy and allows for the repair of damaged mitochondria in infants and adults.
Dr. Minczuk
Faults in our mitochondrial DNA can impact how well the mitochondria function, resulting in mitochondrial disorders, which are serious and frequently deadly ailments that affect approximately one in every 5,000 people. The diseases are incurable and, for the most part, incurable.
Each cell has approximately 1,000 copies of mitochondrial DNA, and the fraction of these that are damaged or altered determines whether or not a person will suffer from mitochondrial disease. More than 60% of the mitochondria in a cell must be faulty for the disease to manifest, and the more defective mitochondria a person has, the more severe their sickness. The condition could potentially be addressed if the fraction of faulty DNA could be lowered.
A ‘heteroplasmic’ cell is one that includes a mix of healthy and defective mitochondrial DNA. A cell is considered ‘homoplasmic’ if it lacks healthy mitochondrial DNA.
In 2018, a team from the MRC Mitochondrial Biology Unit at the University of Cambridge used an experimental gene therapy treatment in mice and were able to successfully target and destroy defective mitochondria DNA in heteroplasmic cells, allowing mitochondria with healthy DNA to take their place.
“Our previous technique was incredibly promising, and it was the first time anyone had been able to modify mitochondrial DNA in a living animal,” Dr Michal Minczuk noted. “It would, however, only function in cells that had enough healthy mitochondrial DNA to duplicate themselves and replace the flawed ones that had been deleted. It would not operate in cells if the entire mitochondrial DNA was defective.”
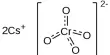
Dr. Minczuk and colleagues employed a biological tool known as a mitochondrial base editor to change the mitochondrial DNA of living mice in their newest advance, which was reported today in Nature Communications. The medication is given into the mouse’s bloodstream by a modified virus, which is subsequently taken up by the mouse’s cells. The tool searches for a distinct sequence of base pairs, which are combinations of the A, C, G, and T molecules that make up DNA. It then alters the DNA base, in this case from a C to a T. In theory, this would allow the tool to repair specific “spelling mistakes” that cause mitochondrial dysfunction.
Because there are currently no adequate mouse models of mitochondrial DNA disorders, the researchers tested the mitochondrial base editors on healthy mice. It does, however, demonstrate that it is possible to modify mitochondrial DNA genes in a living animal.
Pedro Silva-Pinheiro, a postdoctoral researcher in Dr. Minczuk’s lab and the study’s primary author, stated: “This is the first time anyone has been able to modify DNA base pairs in a living animal’s mitochondria. It demonstrates that, in theory, we may go in and rectify spelling errors in damaged mitochondrial DNA, resulting in healthy mitochondria that allow the cells to operate normally.”
A technique developed in the United Kingdom known as mitochondrial replacement treatment, often known as ‘three-person IVF,’ allows a mother’s faulty mitochondria to be replaced with those from a healthy donor. However, this process is complicated, and even regular IVF is only successful in one out of every three rounds.
Dr. Minczuk continued: “Our research clearly has a long way to go before it can lead to a cure for mitochondrial illnesses. However, it demonstrates the possibility of a future treatment that eliminates the intricacy of mitochondrial replacement therapy and allows for the repair of damaged mitochondria in infants and adults.”