Researchers at the University of Arizona Health Sciences are one step closer to developing a non-opioid pain reliever after a study revealed that a new compound they created reduces the sensation of pain by regulating a biological channel associated with pain.
The majority of people will experience pain at some point in their lives, and the National Institutes of Health estimates that 100 million Americans suffer from chronic pain. According to the National Institute on Drug Abuse, approximately 21-29 percent of patients prescribed opioids for chronic pain misuse them, and 8-12 percent of people using opioids for chronic pain develop an opioid use disorder. Nearly 50,000 people died in the United States in 2019 as a result of opioid-related overdoses.
“Drug discovery for chronic pain is at the forefront of this research, and it’s being amplified by the intersection of the COVID-19 pandemic and the opioid epidemic,” said Rajesh Khanna, PhD, associate director of the UArizona Health Sciences Comprehensive Pain and Addiction Center, professor of pharmacology in the UArizona College of Medicine — Tucson, and member of the BIO5 Institute. Our lab investigated a fundamental mechanism of pain, devised a method to distinguish it from those that came before us, and discovered a compound that has the potential to be a new non-opioid pain treatment.”
The study was published today in Science Translational Medicine under the title “Selective targeting of NaV1.7 via inhibition of the CRMP2-Ubc9 interaction reduces pain in rodents.” NaV1.7, a sodium ion channel previously linked to pain sensation in genetic studies of people with rare pain disorders, is the biological mechanism at the heart of the research.
Drug discovery for chronic pain is at the forefront of this research, and it’s being amplified by the intersection of the COVID-19 pandemic and the opioid epidemic. Our lab investigated a fundamental mechanism of pain, devised a method to distinguish it from those that came before us, and discovered a compound that has the potential to be a new non-opioid pain treatment.
Rajesh Khanna
Nerve cells, or neurons, use electrical currents to send signals to the brain and throughout the body, and sodium ion channels are critical to a cell’s ability to generate those electrical currents. When a neuron is stimulated, the NaV1.7 channel opens, allowing positively charged sodium ions to cross the cell membrane and enter the previously negatively charged cell. The change in charge across the cell membrane generates an electrical current, which increases the excitability of the neuron and sets in motion a chain of events that leads to pain.
Because NaV1.7 is a human-validated pain target, several attempts have been made to alleviate pain by blocking NaV1.7 with sodium ion channel inhibitors. None of them have been successful. Dr. Khanna and his colleagues took a different approach, preferring to indirectly regulate NaV1.7 rather than block it.
The team successfully regulated NaV1.7 activation in the laboratory using nerve cells from four different species, including humans, using a compound they designed and dubbed 194. 194 was effective in reversing pain in six different pain models in both sexes in animal models.
Researchers discovered that 194 may also promote pain relief by activating the body’s endogenous, or naturally occurring, opioid system. Endogenous opioids, once produced, activate receptors, resulting in physiological changes such as pain relief. And 194 of them did so without causing motor performance issues, depressive behaviors, or addiction.
Finally, Dr. Khanna and colleagues discovered a synergistic effect when 194 was combined with morphine and gabapentin. This is a promising sign that 194 could be used in a dose-reduction strategy for painkillers with negative side effects, such as opioids, while still providing effective pain relief.
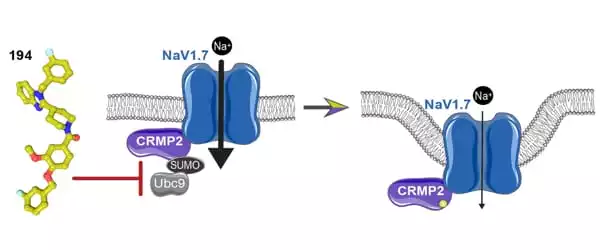
The science behind 194
Dr. Khanna’s previous research identified a protein called collapsin response mediator protein 2 (CRMP2) and an enzyme called Ubc9, both of which play a role in NaV1.7 activation. CRMP2 is a protein that binds to NaV1.7 and transports it to the cell membrane, where sodium ions enter the cell. Ubc9 is an enzyme that tags CRMP2 with another protein – a small ubiquitin-like modifier protein – to specifically control NaV1.7.
Dr. Khanna and his colleagues set out to see if they could directly regulate the activity of NaV1.7 by preventing Ubc9 from interacting with CRMP2. May Khanna, PhD, associate professor of pharmacology and BIO5 Institute member, Vijay Gokhale, PhD, associate research professor in the BIO5 Institute, and Samantha Perez-Miller, PhD, researcher and scientist in the Department of Pharmacology, and their colleagues examined 50,000 existing small molecules to find those with a structure similar to Ubc9.
They chose less than 50 of the closest matches, which were then tested in Dr. Khanna’s laboratory to see if their presence would inhibit sodium influx through NaV1.7. The findings were promising, so the team set their sights on creating a one-of-a-kind, more effective compound.
The result was 194, which UArizona patented and licensed to startup Regulonix LLC through Tech Launch Arizona, the UArizona office that commercializes inventions resulting from university research. Drs. Khanna and Gokhale founded Regulonix LLC in 2016 to address the growing opioid epidemic by developing new, non-addictive pain treatments and commercializing those innovations.
While 194 shows a lot of promise for pain relief, Dr. Khanna and his team have been working with the National Institutes of Health’s National Center for Advancing Translational Sciences to improve the compound. In this case, an NCATS team is primarily concerned with improving 194’s half-life (the amount of time it takes for a drug to reduce by half in your body) and drug-like properties.
It is an important step toward optimizing the compound’s potential as a pain-relieving drug and moving on to the next stage, in which researchers will seek FDA approval to begin clinical trials.














