Kirchhoff’s Laws are applications of two fundamental conservation laws: the Law of Conservation of Energy, and the Law of Conservation of Charge.
(i) The silvered surface of a thermos flask is a bad absorber as well as a bad radiator. Hence, ice inside the flask does not melt quickly and hot liquids inside the flask do not cool quickly.
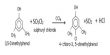
(ii) Sodium vapours on heating emit two bright yellow lines. These are called D1 and D2 lines of sodium. When continuous white light from carbon arc passes through sodium vapour at low temperature, the continuous spectrum is absorbed at two places corresponding to the wavelengths of D1 and D2 lines and appear as dark lines. This is in accordance with Kirchoffs law.