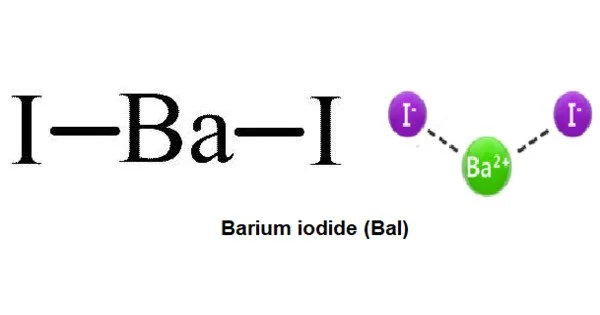Barium iodide is an inorganic compound with the formula BaI2. It obtained by the decomposition of the barium by means of metallic sodium. The compound exists as an anhydrous and a hydrate [BaI2(H2O)2], both of which are white solids. When heated, hydrated barium iodide converts to the anhydrous salt. The hydrated form is freely soluble in water, ethanol, and acetone.
Barium iodide is widely used in the manufacturing of other important compounds of iodine. This compound is also used to prepare barium dioxide.
Properties
Barium iodide crystallizes in small, colorless needles, which deliquesce slightly, and are very soluble in water. The molecular weight of the anhydrous form as determined by the formula of barium iodide is 391.136 g/mol. Similarly, the molar mass of the hydrated form as can be calculated from the barium iodide formula is 427.167 g/mol.
- Chemical formula: BaI2 (anhydrous); BaI2·2H2O (dihydrate)
- Molar mass: 391.136 g/mol (anhydrous); 427.167 g/mol (dihydrate)
- Appearance: White orthorhombic crystals (anhydrous) colorless crystals (dihydrate)
- Odor: odorless
- Density: 5.15 g/cm3 (anhydrous); 4.916 g/cm3 (dihydrate)
- Melting point 711 °C (1,312 °F; 984 K) (anhydrous)l decomposes at 740 °C (dihydrate)
- Solubility in water: 166.7 g/100 mL (0 °C); 221 g/100 mL (20 °C); 246.6 g/100 mL (70 °C)
- Solubility: soluble in ethanol, acetone

Structure
The structure of the anhydrous form resembles that of lead(II) chloride with each Ba center bound to nine iodide ligands and has a crystalline packing structure that is quite similar to BaCl2.
Barium iodide is known to form organometallic compounds that are of particular research interest. Barium Iodide reacts with compounds of alkyl potassium in order to form the corresponding organobarium compounds. Barium Iodide is known to undergo reduction with lithium biphenyl which produces a highly reactive form of Barium as a reaction product.
Chemical Properties
Barium iodide reacts with potassium bromide forming yellow precipitate of potassium iodide and barium bromide.
KBr + BaI2 → KI + BaBr2
Barium sulfate is formed when barium iodide is treated with sodium sulfate. The chemical equation is given below.
BaI2 + Na2SO4 → BaSO4 + NaI
Reactions
Anhydrous BaI2 can be prepared by treating Ba metal with 1,2-diiodoethane in ether.
BaI2 reacts with alkyl potassium compounds to form organobarium compounds.
BaI2 can be reduced with lithium biphenyl, to give a highly active form of barium metal.
BaI2 is neutral salt; it is formed by the reaction of hydrogen iodide and barium hydroxide. Both hydrogen iodide and barium hydroxide are strong acid and base respectively.
Uses
- Used in the preparation of barium dioxide, but has no medicinal use
- Used in the manufacture of other iodide compounds.
- Used to densify the copper castings described in a paper before the Electrochemical Society.
Safety
Like other soluble salts of barium, barium iodide is toxic.