For the first time, doctors used gene therapy to restore partial vision in a blind person. In a man whose vision had been destroyed by retinitis pigmentosa, a genetic disorder that breaks down cells that absorb and convert light into brain signals, the researchers genetically altered retinal ganglion cells to become light-sensitive.
Researchers reported in the June issue of the journal Nature Medicine that the 58-year-old man went from total blindness to being able to detect a large notebook, a smaller staple box, glass tumblers, and even the stripes of a street crosswalk using special goggles.
According to research published in the journal Nature Medicine, the treatment employs RNA molecules that can infiltrate cells and reverse a specific mutation linked to Leber congenital amaurosis, an eye disease that causes blindness in infancy. Surprisingly, the treatment’s long-term effects were discovered largely by chance by researchers at the University of Pennsylvania School of Medicine.
A blind patient regained their vision for over a year after getting a single injection of an experimental gene therapy directly into their eye.
The mutation that causes Leber congenital amaurosis prevents cells from producing the CEP290 protein, which is essential for photoreceptor cells in the eye. The treatment inserts RNA into those cells, causing CEP290 production and effectively reversing the mutation for months at a time.
According to the study’s press release, the researchers first tested this gene therapy in 2019 in an earlier experiment in which patients were injected every three months. Because the RNA was replenished on a regular basis, the patients’ vision improved over time. However, one participant only received the initial injection before withdrawing due to concerns about potential side effects.
And what a lucky break it was for them. The new study focuses solely on that patient and reveals that the restored vision peaked two full months after the injection and lasted for more than 15 months — long-term trends that were obscured by the other patients’ repeated treatments.
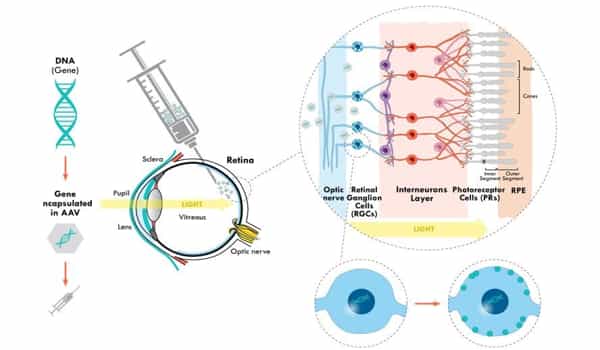
As the study’s authors explain in the press release, this is a promising sign for this specific treatment as well as the field of RNA-based gene therapies in general. Longer-lasting benefits would not only potentially reduce medical costs associated with repeated injections, but would also provide scientists working on other conditions with a promising avenue to explore their own gene therapies.
The study only includes one patient because the pandemic hampered the research team’s ability to train and test other clinical trial participants. “They’re telling us what they’re seeing and how they’re using their restored vision,” Sahel says. “Patients are truly partners in trials now more than ever.”
As the pandemic fades, the researchers hope to begin training other patients enrolled in the trial and evaluating how the goggles help. The project is expected to be completed in late 2025. Beyond restoring vision to those suffering from retinitis pigmentosa, optogenetics is already revolutionizing medical science, and “its full potential is really still being explored,” according to Lewis.
For decades, scientists have used electrical stimulation to coax nerve cells to activate or deactivate. However, electrical stimulation is not sufficiently controlled. It’s like attempting to direct a lightning bolt to the brain. They stimulate a region rather than specific cells.
Researchers can use optogenetics to genetically engineer specific nerve cells to respond to light stimulation. This allows for more precise control over cell activity and allows researchers to study the brain with greater accuracy. Precision is essential. Using the technique, scientists have been able to regulate motor behavior in animal models of Parkinson’s disease. Using light to change the specific activity of nerve cells could provide a therapeutic strategy to combat the disease.