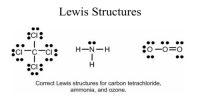
The electronic configurations of sodium and chlorine arc as follows:
Na (11) → 1s2 2s2 2p6 3s1
Cl (17) → 1s2 2s2 2p6 3s2 3p5
There is one electron in the outermost shell of sodium. On the other hand, there are seven electrons in the outermost shell of chlorine, i.e., one less than the inert gas. When sodium atom and chlorine atom come near each other, sodium atom donates one electron to chlorine atom, thereby forming singly charged positively sodium ion (Na+) and singly negatively charged chloride ion (Cl–). Their electronic configuration, resemble the electronic configurations of neon and argon respectively. These two ions are attracted to each other and remain in close contact. Thus sodium chloride is formed process can be represented as follows:
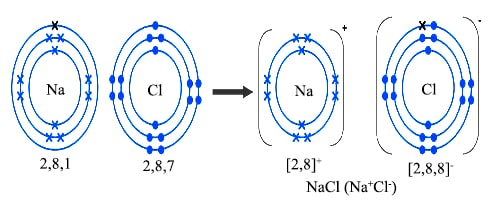
Fig: Formation of NaCl