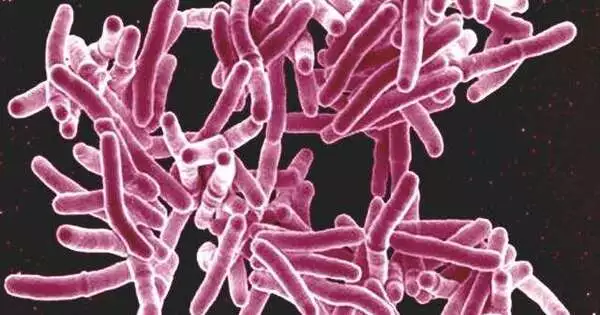
A collaborative team of scientists has solved the molecular cause of a strange outbreak of tuberculosis that exited the lungs and began chewing away at bones 15 years ago in North Carolina. The mysterious bug reverted to an ancestral form, which increased the mobility and activity of its macrophage hosts.
Tuberculosis is most commonly associated with the lungs, but in 2% of cases in the United States, it can also be found in the bones. Some 9,000-year-old Egyptian mummy skeletons show signs of tuberculosis infection in their bones, a painful condition that causes the bones to look gnawed.
So it was a strange puzzle when Duke physician Jason Stout M.D. encountered a Wake County TB outbreak in the mid-2000s in which the infection had spread beyond the lungs in six people. “Four out of six were in the bone,” Stout said. “That’s far more than 2%.”
The index case, the first person in Raleigh to have this strain of the disease, apparently contracted the bacterium in Vietnam, but he wasn’t feeling too ill and had been working with 400 other people at his workplace.
“So it was prolonged exposure in the workplace,” said Stout, a Duke professor of medicine who tracked down and identified seven subsequent infections using contact tracing and health department records.
We met up and we’re having coffee one day, and we’re talking about this. Well, give it to me and we’ll take a look. And then this amazing science came from that.
David
All eight people were treated with antibiotics and other co-workers received preventative care and then the strange outbreak went away. But the mystery was never really solved. “I’m an epidemiologist and clinical trial specialist and I was left scratching my head,” Stout said.
Until several years later when Stout had a chance conversation with his colleague and TB researcher David Tobin, Ph.D., an associate professor in molecular genetics and microbiology and immunology at Duke.
“We met up and we’re having coffee one day, and we’re talking about this,” Stout recalls. Academic medical centers like Duke routinely keep biological specimens, and Stout still had samples of the puzzling bug. “David said, ‘Well, give it to me and we’ll take a look.’ And then this amazing science came from that,” Stout said.
Tobin’s lab, along with colleagues from Duke, Notre Dame, and the University of Texas, figured out exactly how and why these particular TB bacteria were so mobile. Their findings were published online in the journal Cell on November 9th.
“Some infections tend to go to specific places,” Stout explained. “And the question is always why it does that?” The bacteria in TB strains from the Americas and Europe appear to be more likely to remain in the lungs. However, this strain was extremely mobile.
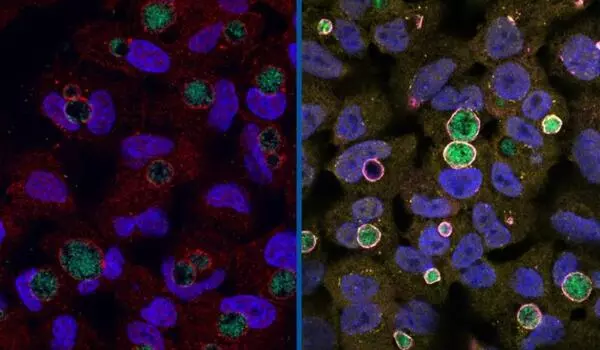
Tobin’s team, led by Joseph Saelens, Mollie Sweeney, and Gopinath Viswanathan, ran genetic sequencing on the Raleigh bug and found it most resembled an ancestral strain from a group of strains called lineage 1. In the U.S. we tend to see the modern strains, lineages 2, 3, and 4, but lineage 1 is still out there, mostly in South and Southeast Asia.
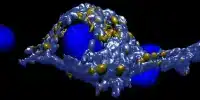
Mycobacterium tuberculosis typically infects a macrophage, a highly mobile street sweeper cell that moves around looking for invaders and then engulfs and chews them up. One component of the pathogenic bacteria’s toolkit is a set of unique chemical signals — secreted factors — that it uses to protect itself from the immune system and communicate with its macrophage host.
Tobin’s team was looking for the difference that allowed the macrophages of the Wake County bug to move around and leave the lungs. They compared genetic variants from 225 different strains of tuberculosis, paying special attention to genes for secreted factors. What they found is a secretion factor called EsxM that was active in the Raleigh bacteria, but had been inactivated by a mutation in the modern strains.
Then, working with Craig Lowe, an evolutionary biologist and assistant professor of molecular genetics and microbiology at Duke, they looked at genetic sequencing from 3236 different strains of TB and found the pattern persisted: the modern strains have a silenced version of the EsxM secretion factor. “Over a few thousand strains, that really holds up,” Tobin said. “They’ve maintained that and presumably, it’s something that’s evolutionarily advantageous to them.”
To further demonstrate their point, the researchers injected active EsxM into attenuated versions of modern strains and observed how their macrophage hosts in a lab dish became more active and mobile. “We can see changes in macrophage shape and structure as they migrate,” Tobin explained. They also knocked out EsxM in a strain with the ancestral version, which reduced the mobility of infected macrophages.
Tobin, while cautioning against overstating their findings, stated that it appears that the widely distributed modern strains of tuberculosis benefit from staying within the lungs due to the way they spread through the air by breathing. Staying in the lungs would presumably provide them with a better starting point for a new host.
Fortunately, the migratory TB strain has not been seen locally since, according to Stout, “hopefully because we did good work and got a lot of people preventative therapy.” However, the mystery of its unusual mobility has been solved.