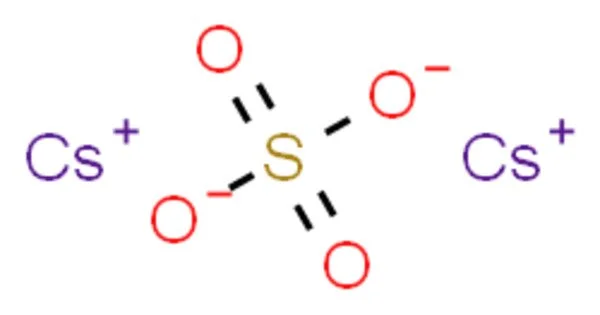
Caesium sulfate, also known as cesium sulfate, is an inorganic compound with the formula Cs2SO4. It’s a white water-soluble solid used to make dense aqueous solutions for isopycnic (or “density-gradient”) centrifugation. With potassium salt, it is isostructural. It is a necessary metal for all living organisms. It is used in the preparation of alloys as a deoxidizer, desulfurizer, and decarbonizer due to its chemical properties. However, it is not just the metal itself; a variety of calcium compounds are also important in a variety of industries and are produced on a large scale.
Cesium sulfate is used in the preparation of dense aqueous solutions for isopycnic centrifugation. It is also used in the formation of crystals.

Properties
- Chemical formula: Cs2SO4
- Molar mass: 361.87 g/mol
- Density: 4.243 g/cm3, solid
- Melting point: 1,010 °C (1,850 °F; 1,280 K)
- Solubility in water: 167 g/100 ml (0 °C)
- Solubility: insoluble in ethanol, acetone
- Magnetic susceptibility (χ): -116.0·10−6 cm3/mol
Applications
Cesium sulfate is used in equilibrium density gradient centrifugation, which is one of the most useful tools for fractionating and analyzing macromolecules like DNA. Cesium sulfate can be used as a catalyst; for example, a cesium sulfate/vanadium pentoxide catalyst is used in abiabatic multibed reactors to lower inlet temperature, and a cesium sulfate/zeolite catalyst is used in a pilot plant scale process to synthesize 4-methyl thiazol.