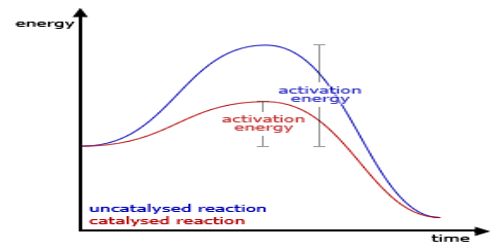
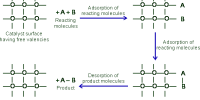
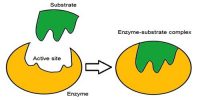
A catalyst cannot, in general, start a reaction. Some reactions, however, cannot start in absence of the catalyst. An example is the reaction between H2 (g) and O2 (g) which can be kept together at room temperature for many years without reaction taking place. The presence of traces of platinized platinum, however, starts the reaction very quickly.
Some scientists believe, mainly from experimental studies, that a catalyst can start a reaction. One way of reconciling the opposite views is to consider the reaction rate to be infinitely slow. The case is, however, more complicated. In such reactions the catalyst at first reacts chemically with one of the components forming a highly reactive species, which then brings about the chemical reaction. In absence of the catalyst the reaction cannot occur. This class of catalysts should be termed ‘initiators’. An example is the use of organic peroxides as initiators in polymerization reactions the peroxide is thought to split into free radicals which initiate the reaction-
ROOR → 2RO•
RO• + CH2 = CH2 → ROCH2CH2•
ROCH2CH2• + CH2 = CH2 → ROCH2CH2CH2CH2•