Catalytic poisons: Substances which, in their presence, reduce the catalytic activity of a catalyst are called catalytic poisons. According to the collision theory, a reaction occurs on account of effective collisions between the reacting molecules. For effective collision, it is necessary that the molecules must possess a minimum amount of energy known as activation energy (Ea). After the collision molecules form activated complexes which dissociate to yield the product molecules. The catalyst provides a new pathway involving lower amount of activation energy. Catalyst poison, substance that reduces the effectiveness of a catalyst in a chemical reaction. In theory, because catalysts are not consumed in chemical reactions, they can be used repeatedly over an indefinite period of time.
Examples are:
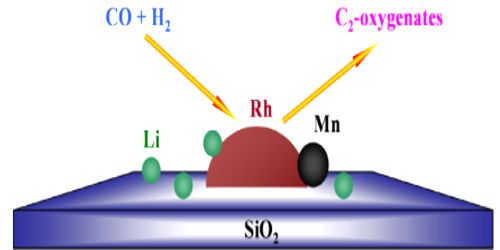
(i) Carbon monoxide gas acts as a poison when present during the hydrogenation of alkenes with finely divided nickel as a Catalyst.
(ii) The catalytic activity of platinum in the oxidation of SO2 to SO3 in the contact process for the manufacture of sulphuric acid is poisoned by the presence of arsenic, dust particles etc.