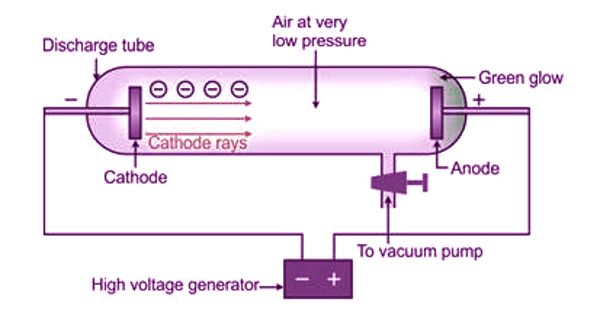
Cathode rays are streams of electrons observed in vacuum tubes. These rays are invisible but their effect is to excite atoms in the glass opposite of the cathode, by the anode. These rays come from the cathode because the cathode is charged negatively. If an evacuated glass tube is equipped with two electrodes and a voltage is applied, the glass opposite the negative electrode is observed to glow from electrons emitted from the cathode. Cathode rays can be deflected by an electric field, which is evidence of it being composed of electron particles rather than photons. These rays focused on a hard target (anticathode) produce X-rays or focused on a small object in a vacuum generate very high temperatures. The rays of electrons can also pass through the thin metal foil. However, cathode rays also exhibit wave-like characteristics in crystal lattice experiments.
J.J.Thomson discovered a new subatomic constituent of cathode rays in the year 1897. He used the cathode ray tube to determine that atoms had small negatively charged particles inside of them, which he called “electrons.” These rays are so named because they are emitted by the negative electrode, or cathode, in a vacuum tube. The cathode rays are deflected from their straight-line path by both electric and magnetic fields. The direction of deflection shows that they are negatively charged particles.
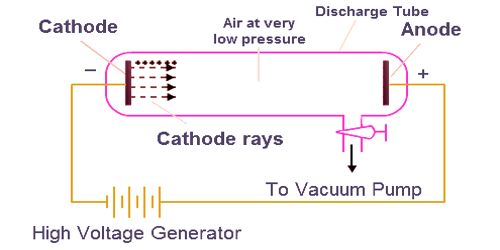
Fig: Observations in the cathode ray tube experiment
Cathode rays have the following properties: The properties of the cathode rays do not depend on the electrodes and the gas used in the vacuum tube.
- They travel in straight lines.
- These rays exert mechanical force on the objects they encounter and strike.
- These rays possess momentum and kinetic energy.
- These rays produce heat when allowed to fall on the matter.
- These rays produce fluorescence.
- These rays produce fluorescence when they strike a number of crystals, minerals, and salts.
- When cathode rays strike a solid substance of large atomic weight, X-rays are produced.
- These rays can ionize the gases through which they are passed.
- These rays affect photographic plates.
- These rays can penetrate through the thin foils of metal.
- These rays comprise of electrons which are fundamental constituents of all atoms.
- These rays are 1800 times lighter than hydrogen, the lightest element.
Application
A cathode-ray tube is a device that uses a beam of electrons in order to produce an image on a screen. The most popular commercial application of cathode ray technology is in the form of traditional television sets and computer monitors, although these are being supplanted by newer displays such as OLED. Food and Drug Administration regulations in 21 CFR 1020.1 are applicable to CRT emissions. They limit CRT radiation exposure to 0.5 mR/hr at a distance of two inches.