General properties of Diamagnetic Material
The materials which have net magnetic moments i.e., those materials which reveal para and ferromagnetism, the diamagnetism in those materials becomes overshadowed due to its weak value. So, Diamagnetism is a quantum mechanical effect that occurs in all materials; when it is the only contribution to the magnetism, the material is called diamagnetic. In diamagnetic materials, there are no atomic dipoles due to the pairing between the electrons. Electrons in an atom revolve around the nucleus thus possess orbital angular momentum. The resultant magnetic momentum in an atom of the diamagnetic material is zero.
In short, it can be said that that material which, when placed in a magnetic field acquires a small amount of magnetism opposite to the direction of the inducing magnetic field are called diamagnetic materials. Examples – copper, silver, zinc, bismuth, lead, glass, marble, helium, water, argon, sodium chloride, etc.
Properties of Diamagnetic Materials –
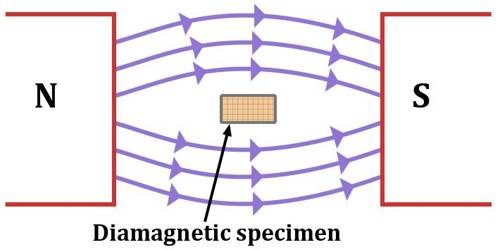
(i) Diamagnetic materials tend to move from a strong intensity to a weaker region in a non-uniform magnetic field. That means there are feebly repelled by the magnetic field.
(ii) There are no atomic dipoles in diamagnetic materials because the resultant magnetic moment of each atom is zero due to paired electrons.
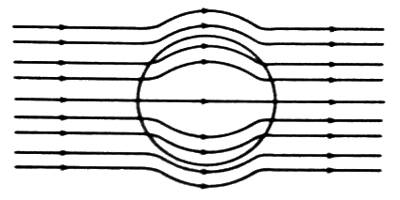
(iii) When diamagnetic material is placed in a magnetic field, the lines of force tend to move away from the material. As a result, a number of lines of force passing through the material become less [Figure].
(iv) Magnetic induction B of a diamagnetic material is slightly less than the magnetic field intensity H.
(v) The substances are weakly repelled by the field so in a nonuniform field, these substances have a tendency to move from a strong to a weak part of the external magnetic field.
(vi) The value of magnetic susceptibility is very small. The intensity of magnetization I is very small, negative, and proportional to the magnetizing field.
(vii) Diamagnetic materials are slightly repelled by a magnetic field and the material does not retain the magnetic properties when the external field is removed.
(viii) Susceptibility of a diamagnetic material does not depend on the applied magnetic field and temperature. In diamagnetic materials all the electron are paired so there is no permanent net magnetic moment per atom.
Properties of diamagnetic substances
- Diamagnetic substances are those in which the net magnetic moment of atoms is zero.
- The susceptibility has a low negative value. (For example, for bismuth χm=? 0.00017).
- Susceptibility is independent of temperature.
- The relative permeability is slightly less than one.
- When suspended freely in a uniform magnetic field, they set themselves perpendicular to the direction of the magnetic field. Examples: Bi, Sb, Cu, Au, Hg, H2O, H2, etc
When an external magnetic field is applied, dipoles are induced in the diamagnetic materials in such a way that induced dipoles opposes the external magnetic field according to Lenz’s law. Diamagnetic properties arise from the realignment of the electron paths under the influence of an external magnetic field. Most elements in the periodic table, including copper, silver, and gold, are diamagnetic.