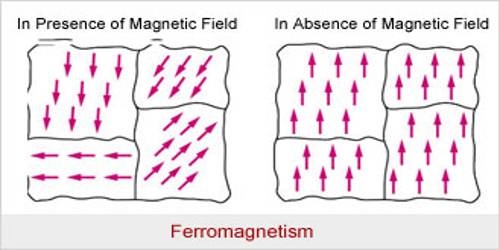
Ferromagnetism is the characteristic of substances such as iron, nickel, or cobalt and various alloys that demonstrate particularly high magnetic permeability, a characteristic saturation point, and magnetic hysteresis. Ferromagnetic materials also belong to the paramagnetic family. But the values of magnetic permeability are many times more and the magnetic attraction is very strong. In these materials, magnetic moments of the paramagnetic atoms or ions remain locked in a large area or region. These small areas or regions are called domains. This type of region contains 1016 – 1019 number of nearby atoms or ions.
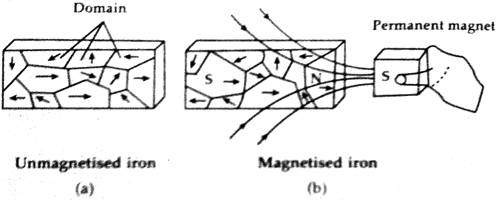
Even the thermal vibration which tends to non align cannot break the locking or alignment up to a particular temperature. The distribution or arrangement of the associated or surrounding atoms or ions is a quantum process which cannot be explained by classical physics. Amongst die atoms or ions in each domain of this type of material there exists a quantum mechanism known as ‘exchange integral’ which is effective inside the domain that keeps the magnetic moments parallel to each other. An unmagnetized ferromagnetic material normally does not show any resultant magnetic moment, the reason for this is that the different domains are distributed randomly [Fig. (a)]. As a result, the collective or resultant moment becomes zero. When these materials are placed in a magnetic field or are brought near a magnet the sizes of slime domains increase in the direction of the field and sizes of some other domain decrease. So, magnetism is induced and external magnetism is exhibited [Fig. (b)].
In short, it can be said that those materials when placed in a magnetic field acquire strong magnetism in the direction of the magnetizing field are called ferromagnetic materials. Examples— Iron, nickel, cobalt etc.