Dia-magnetism
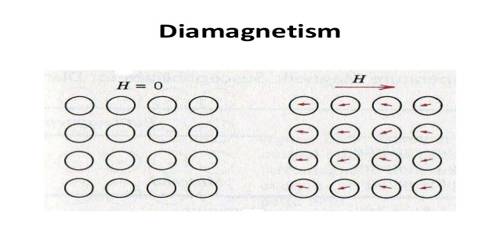
Due to the orbital motion of the electrons in atom diamagnetism appears in the material. Diamagnetism exists in all materials. But its effect is very weak. The materials which have net magnetic moments i.e., those materials which reveal para and ferromagnetism, the diamagnetism in those materials becomes overshadowed due to its weak value. So, Diamagnetism is a quantum mechanical effect that occurs in all materials; when it is the only contribution to the magnetism, the material is called diamagnetic.
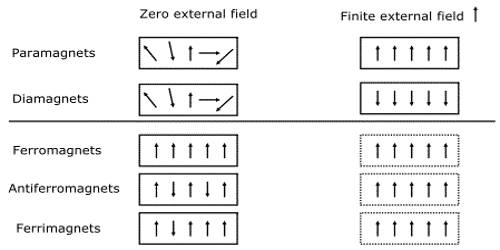
We know that with each revolving electron there is an orbital magnetic moment. But due to the difference in orientation of different orbits of the atom no net orbital moment exists. Magnetic influence of each electron cancels each other. That means, there is no permanent magnetic moment of a diamagnetic substance. In paramagnetic and ferromagnetic substances the weak diamagnetic force is overcome by the attractive force of magnetic dipoles in the material. All materials are actually diamagnetic, in that a weak repulsive force is generated by in a magnetic field by the current of the orbiting electron. Some materials, however, have stronger paramagnetic qualities that overcome their natural diamagnetic qualities.
Some Diamagnetic Elements: Bismuth, Mercury, Silver, Carbon, Lead, Copper etc.
Some Ferromagnetic Elements: Iron, Nickel, Cobalt, Gadolinium, Dysprosium etc.
Some Paramagnetic Elements: Uranium, Platinum, Aluminum, Sodium, Oxygen etc.