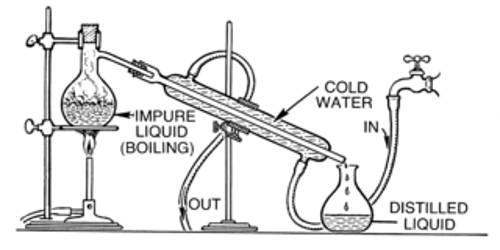
Absolute alcohol can be prepared from rectified spirit by azeotropic distillation. Rectified spirit contains 95.6% ethanol and 4.4% water. This mixture of ethanol is known as an azeotropic mixture. Absolute alcohol is 100% pure alcohol. It is a constant boiling mixture having boiling point 78.130C. The percentage of ethanol cannot be increased by using the distillation method. Because when the mixture boils the composition of the components remains the same in the vapor state. But if we change this composition by adding another component then rectified spirit can be converted to absolute alcohol by distillation method. Here if we add lime (CaO) to the rectified spirit then the water of the mixture can be absorbed by the lime. As a result, the percentage of ethanol will be changed. So the mixture will not act as an azeotropic mixture. In this way, we can prepare absolute alcohol by using the distillation method from a rectified spirit.
Rectified spirit is high concentration alcohol purified by the procedure of rectification. Absolute alcohol normally refers to purified ethanol, containing no more than one percent water. It is used for medicinal purposes, as a household solvent, and in mixed drinks. Neutral grain spirit is a kind of rectified spirit, formed from grain. To attain a pure form of absolute alcohol, one more separation step is required, so that the water content in the rectified spirit is removed completely. It is not probable to obtain absolute alcohol by simple fractional distillation, because a mixture containing around 95.6% alcohol and 4.4% water becomes a constant boiling mixture. In one common industrial method to obtain absolute alcohol, a small quantity of benzene is added to the rectified spirit and the mixture is then distilled. Absolute alcohol is obtained in the third fraction that distills over at 78.2°C (351.3 K). Because a small amount of the benzene used remains in the solution, absolute alcohol produced by this method is not suitable for consumption as benzene is carcinogenic.
Example – Anhydrous Salt Method to Produce Absolute Ethyl Alcohol from Rectified Spirit:
Glycerine well known as a dehydrator is used to remove the water content in rectified spirit turning it into absolute alcohol. In small or medium scale industries, an anhydrous salt method is selected as a choice of operation. Absolute ethanol vapors collected at the top of the scrubbing column. They are condensed to a liquid. The bottom stream of the scrubber contains a mixture of glycerine, water, and traces of alcohol. This bottom mixture sent to a distillation column to acquire dehydrated glycerine and recycle it to the scrubber.