Component (C): The number of component is defined as –
The minimum number of independently variable chemical entities required to describe all parts of a system.
These chemical entities may undergo chemical reaction or physical changes or both, with an increase or decrease of different entities; the number of phases may also change depending on the nature of the changes. To define the equilibrium conditions of the system it will not be necessary to consider all the chemical entities. There can be systems of one, two or more components. Few examples are given below:
One component systems:
Water: Water may exist in three different phases- solid, liquid and vapour. In each case the chemical entity is H2O.
Sulphur: Sulphur can exist in four phases — two allotropes (a) monoclinic (s) and (b) rhombic as a liquid and as vapour. Again in all these phases the chemical constituent is the same.
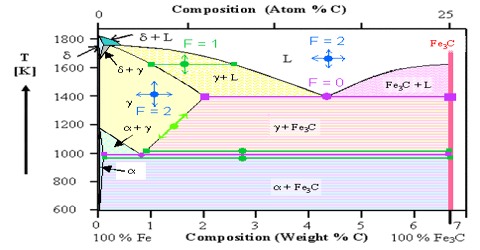
Two component systems:
(a) If CaCO3 is heated in a closed container it undergoes dissociation forming CaO and CO2. When the system attains equilibrium all three chemical substances are present. These three chemical entities are related by the equation –
CaCO3 (s) → CaO (s) + CO2 (g).
There will be three phases, each of the chemical entities representing a phase as they are bounded by a separate boundary, but the number of components will be two because all the three phases can be described by mentioning any two of them as they are related by the above equation.
(b) A solution of NaCl is a one phase system. The number of components in the system is, however, two as the concentration can vary and the composition of the system cannot be described by mentioning any one of them. Similarly the number of components in a saturated solution of the salt is also two for the same reason.
(c) Systems in which salts form one or more hydrates are two component systems as the composition of all phase (hydrates) and water can be described by the constituents, e.g., the salt and water.
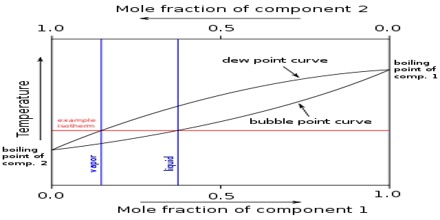
(d) Pairs of partly miscible or immiscible liquid.
(e) Pairs of metals which are miscible in the liquid phase but form separate pure solid metal phases.