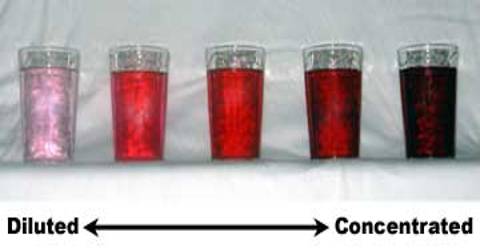
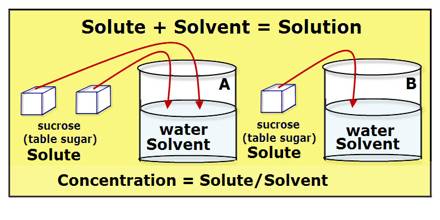
Concentration of solution: Dilute and concentrated solution.
Solution of different concentrations can be produced by mixing more or less solute and solvent in the solution. The solution is called dilute or concentrated depending upon the concentration level of the solution. Now, let us make solutions of different concentrations.
Task: To make concentrated and dilute solution and differentiate them.
Required accessories: Two glass beakers, a stiffer, measuring flask, spoon, sugar and water.
Procedure: Clean the two beakers by washing. Take 100 milliliters of thinking water in beaker using a measuring flask. Pure 1 spoon of sugar in one beaker and 3 spoons of sugar in the other one and stir them gently. Now test the sweetness of the solution of sugar juice by taking 1 spoon from each beaker. (The majority of chemical substances are injurious to human-body. So it is not wise to test by drinking or eating any solution or chemical substances without thoroughly knowing about them.)
The beaker in which 3 spoons of sugar were added has the mixture that test sweeter. Like sugar juice, out of the two solutions of same volume the solution which one has comparatively more amount of solute is called concentrated solution and the other solution which has comparatively less amount of solute called dilute solution.
Again if it happens so that keeping the amount of sugar (1 spoon) the same in both the beakers the amount of water is increased, the solution in the beaker having less amount of water will be sweeter. In this case too, the solution with less amount of water is concentrated solution. And the beaker in which the amount of water is more will be less sweet and we may call this dilute solution.