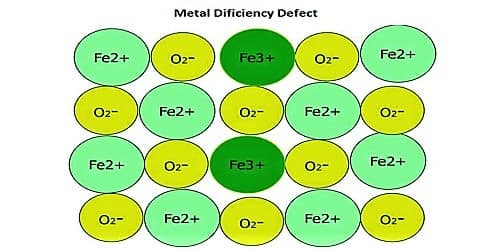
Metal Deficiency Defects: In certain cases, one of the positive ions is missing from its lattice site and the extra negative charge is balanced by some nearby metal ion acquiring additional charges instead of the original charge. This type of defect is generally found in compounds of transition metals which can exhibit variable valency. FeO and FeS show this type of defects.
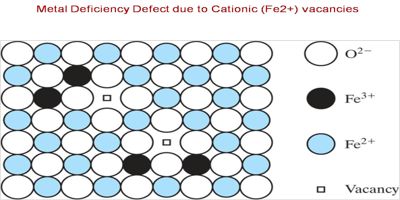
The crystal with metal shortage defect is Ferrous Oxide. This is represented by the chemical formula FeO. The metal shortage in ferrous oxide arises because the constructive ion in the crystal is smaller than the negative ions. This inorganic compound appears in the shape of black crystals that is impenetrable in water, alcohol, and alkali but is dissolved in acid. In this, a cation is missing from its lattice site.
In some cases, the positive ions may be absent from their lattice sites. To continue electrical detachment, one of the adjacent metal ions acquires two positive charges. The extra negative charge might be evenhanded by various close metal ion acquiring two positive charges in its place of one. This type of deficiency is probable in metals which explain variable oxidation states.
It is obvious from the above discussion that all types of point defects consequence in the formation of vacancies or ‘holes’ in the lattice of the crystals. The occurrence of holes lowers the density as well as the lattice energy or the constancy of the crystals. The presence of too many holes might reason a fractional fall down of the lattice.