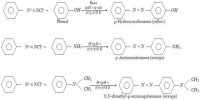
Here explain difference properties between Organic Compounds and Inorganic Compounds:
Organic compounds
- Number: About 8.2 million organic compounds are found.
- Composition: They contain carbon as an essential constituent along with a few other elements like N, 0, S, P, halogens ant a few metals
- Nature: These Compounds are covalent.
- Classification: Organic compounds are classified into many classes according to the functional groups contained in them.
- Melting and boiling points: Organic compound possess low melting and boiling points (less than 300°C or 330°C) and are mostly flammable. E.g. the melting point of oxalic acid is 101°C.
- Solubility: These are generally insoluble in water but soluble in organic solvents like alcohol, ether, benzene etc.
Inorganic compounds
- Number: Total number of inorganic compounds is about one lac.
- Composition: Inorganic compounds are formed from any of the known clement. Example: HCL. CO2 etc.
- Nature: These are ionic compounds.
- Classification: Inorganic compounds do not include many classes. Mostly they are acids, bases and salts.
- Melting and boiling points: They possess high melting and boiling points e.g melting point of NaCl is 801°C.
- Solubility: They are generally soluble in water insoluble in organic solvents.