The modern periodic law states that, “The physical and chemical properties of the elements and their compounds are periodic functions of their atomic number.”
In modern periodic table, elements are arranged according to their increasing atomic numbers.
Structure:
The modern periodic table consists of horizontal rows called periods and vertical columns called groups. These are discussed below:
Periods:
A horizontal row in the periodic table is called periods. In terms of electronic structure of an atom a period constitutes a series of elements whose atoms have the same number of electron shells. i.e. principal quantum number (n). There are seven periods—
- The first Period: Corresponding to n=1 is unique because it contains only two elements. This is because first energy shell has only one orbital, which can accommodate only two electrons. It contains Hydrogen (1s1) and Helium (1s2).
- The second Period: contains 8 elements because of n=2, there are four orbital (one 2s and three 2p). These four orbital have a capacity of eight electrons. Therefore, the second period has 8 elements. It starts from lithium (z=3) and ends at neon (z=10).
- The third Period: Corresponding to n=3, there are nine orbital one 3s, three 3p and five 3d. 3d-orbital are filled after filling 4s-orbital. Hence, this period involves the filling of only four electron (3s and 3p) and contain eight elements from sodium (z=11) to argon (z=18).
- The fourth Period: Corresponding to n=4 involves the filling of one 4s and three 4p-orbital. In between 4s and 4p-orbital, five 3d-orbital are filled which have high energy in between these orbitals. Thus, in all nine orbital are 0 to be filled, therefore, there are eighteen elements in the fourth period from potassium (z=19) to krypton (z=36).
- The fifth Period: Like the fourth period it also consists of 18 elements. If begins with rubidium (z=37) to Xenon (z=54).
- The sixth Period: Contains 32 elements, (z=55 to 66) and successive electrons enter into 6s, 4f, 5d and 6p orbital. It starts from cesium (z=55) and ends at radon (z=86).
- The seventh period: Though expected to have 32 elements, it is incomplete and contains only 24 elements at present.
Groups:
The vertical columns in the periodic table are called groups. There are 18 groups in the periodic table. The groups Ito VII are divided into sub-groups A and B. The groups are numbered as IA, IIA, IIIA, IVB, VB, VIB, VIIB, VIII, IB, IIB, IIIA, IVA, VA, VIA, VIIA and zero groups. All the elements in a group have similar properties.
The first two groups on the extreme left and last six groups on the extreme right involve the filling of s- and p-orbital respectively. These groups represent the main groups of periodic table and are numbered as 1, 2, 13, 14, 15, 16, 17 and 18 corresponding to general configuration of ns1, ns2, ns2np1, ns2np2, ns2np3, ns2np4, ns2np5 and ns2npb respectively.
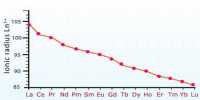
There two more rows at the bottom of the table. These rows consist of 14 elements after lanthanum (z=57) and fourteen elements which follow actinium (z=89). These are lanthanides and actinides and are all together called inner transition elements or rare earth metals.