Energy Changes in Catalytic Reactions
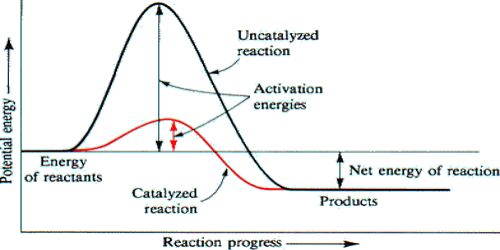
The question may arise as to how does the catalyst enhance the rate of reactions? This can be explained with the help of an energy diagram as in Figure. It has been shown that a catalyzed reaction has lower activation energy than the same reaction taking place in the absence of the catalyst. This is possible if the catalyst provides an alternative route for the reaction with lower activation energy. Figure shows that the enthalpy change for the reaction, H reaction, is not affected by the presence of the catalyst. It should be recognized that the catalyst does not lower the activation energy. A catalyst provides a different route with lower activation energy.
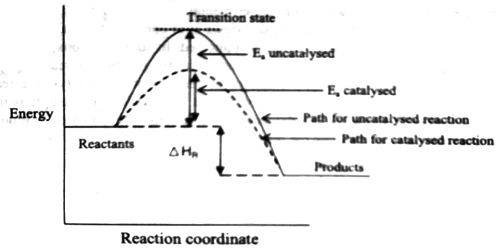
Fig: Energy diagram for unanalyzed and catalyzed reactions.
In Figure, the unanalyzed reaction is shown as a single step with high energy of activation. The catalyzed reaction is shown as a two-stage process with lower activation energy and is, therefore, faster. It is possible that the unanalyzed reaction proceeds by more than one step and the catalyzed reaction may also consist of several steps. The important point to note is that for the catalyzed reaction there is no step which has higher activation energy than the unanalyzed reaction. It may be noted that the ΔHReaction is the same (Figure), since energies of the reactants and the products remain unchanged in the presence of the catalyst.
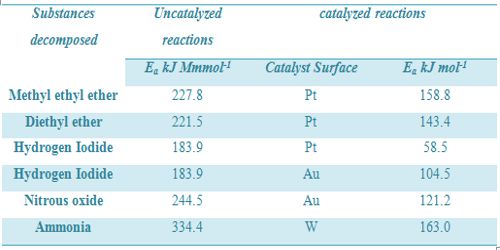
If, however, the reaction is reversible, the catalyst equally decreases the energy of activation of the forward reaction and reverse reaction. Thus, the rate of the forward reaction is accelerated to the same extent as that of the reserve reaction. As a result the equilibrium is quickly established, but the position of equilibrium remains unchanged. Effect of catalyst on some reactions is given in Table.