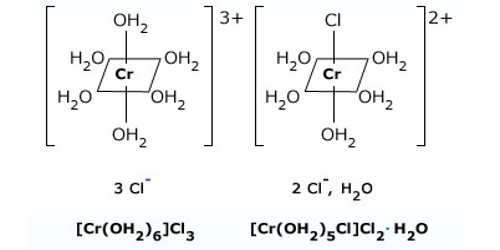
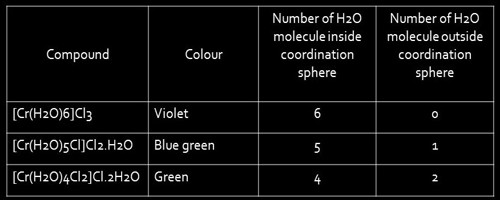
Solvate or hydrate isomerism are the isomers that have similar composition but differ with respect to the number of solvent ligand molecules as well as the counter ion in the crystal lattice. The best-known examples of Hydrate Isomerism or Solvate Isomerism type of isomerism occurs for chromium chloride “CrCl3.6H2O” which may contain 4, 5, (or) 6 coordinated water molecules.
- [Cr(H2O)4Cl2]Cl.2H2O – Bright green
Tetraaquadichlorochromium(III) chloride dihydrate
- [Cr(H2O)5Cl]Cl2.H2O – grey-green
Pentaaquachlorochromium(III) chloride monohydrate
- [Cr(H2O)6]Cl3 – Violet
Hexaaquachromium(III) chloride
These isomers have very different chemical properties and in reaction with AgNO3 to test for Cl- ions, would find 1,2, and 3 Cl- ions in solution respectively.
They differ in that one ion is directly attached to the vital metal but the other is not. A very comparable type of isomerism results from the replacement of a coordinated group by a solvent molecule (Solvate Isomerism). Ionization isomers are coordination compounds that have 2 different ligands swapped between the inner and the outer coordination spheres. In the special case when one of these ligands is a water molecule, the isomerism is called hydrate isomerism. They differ in that one ion is directly attached to the central metal but the other is not. To take an example, [CoBr(H2O)5]Cl and [CoCl(H2O)5]Br are ionization isomers since the chloride and bromide ligands have got mutually swapped. A very similar type of isomerism results from the replacement of a coordinated group by a solvent molecule (Solvate Isomerism). Typically, the water molecules in the outer coordination sphere are shown as a water of crystallization.