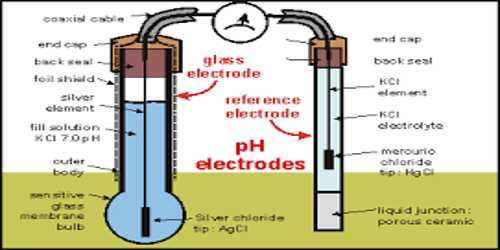
The glass electrode for pH Measurement
Glass electrode has become the most useful and convenient electrode in determining the pH of solutions, particularly with the commercially built pH meters. Most often used pH electrodes are glass electrodes. A typical model is made of glass tube ended with small glass bubble. A glass electrode consists of a glass tube with a thin-walled bulb at the end (Figure). Inside of the electrode is usually filled with a buffered solution of chlorides in which silver wire covered with silver chloride is immersed. pH of internal solution varies. The bulb is filled with 1 mol L-1 HCl solution. One end of a silver wire is coated with AgCl and sealed into the glass bulb in such a way that the AgCl-coated end dips into the HCl solution. The entire assembly forms an electrode known as glass electrode. This is represented as-
Ag│AgCl│HCl (1 mol L-1)│Glass│
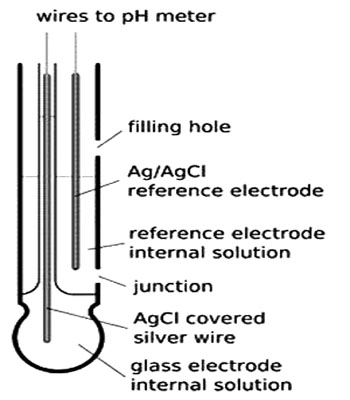
If the glass electrode is placed in a solution of H+ ions, a potential is developed across the glass membrane due to the difference of the H+ ion concentration on the two sides of the membrane. The potential is given by,
EGlass = E0Glass – RT/F ln [H+]
= E0Glass – 0.0591 pH … …. … (1)
In order to determine the pH of an unknown solution the glass electrode is combined with calomel electrode to set up the following cell:
Glass Electrode│Solution of unknown pH│Calontel Electrode
The emf of the cell is given by,
Ecell = Ecal – EGless …. …. (2)
Substituting equation (1) into (2) we get,
Ecell = Ecal – EGless – 0.0591 pH
pH = (Ecal – EGless – Ecell) / 0.0591
Thus the pH of an unknown solution can be easily determined by measuring the e.m.f. of the cell, since, ECal and E0Glass are known. Glass electrodes are commercially available. These have the following advantages:
(i) simple to use,
(ii) not affected by oxidizing and reducing agents,
(iii) not easily poisoned,
(iv) can be used for a few solutions.
However, glass electrodes are very delicate and should be handled carefully. The glass electrode is an essential part of the pH-meters which are widely used in biological studies, in industries, and in the analysis.