Measurement of pH
pH measurement is used in an extensive diversity of applications: agriculture, wastewater treatment, industrial procedures, environmental monitoring, and in research and development. pH is a measure of the acidity or alkalinity of a solution.
The methods of determination of pH of a solution are broadly divided into two groups:
(a) the indicator method and
(b) the e.m.f. method
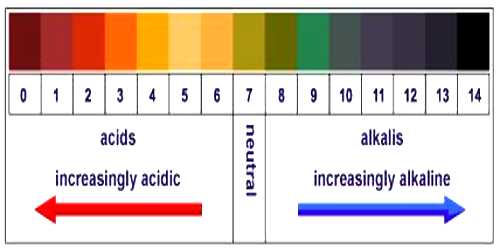
Indicator method: This method is based on the fact that indicators have different colors in solutions of different pH. From previous knowledge of the color-pH match, one can directly determine the pH of a solution by adding one or two drops of the indicator solution. A suitable mixture of different indicators, known as universal indicator, can help determine the pH of a solution over a wide range of values. Nowadays, special papers, known as pH-papers, are also available for approximate determination of pH of a solution. The indicator method is simple and easy to use but provides approximate value only of pH of the solution.
The e.m.f. method: This as based on the use of an electrode whose potential is dependent on H+ ion concentration in solution. Three such electrodes are usually used. These are
(i) the hydrogen electrode,
(ii) the quinhydrone electrode and
(iii) the glass electrode.
Each of these electrodes is combines, with a reference calomel electrode to set up a cell and the e.m.f. of the cell is determined to evaluate accurately the pH of the solution.