In a complex compound, it usually, central metal ion and the ligands are enclosed within a square bracket is called a coordination sphere. This represents a single constituent unit. The ionizable species are placed outside the square bracket.
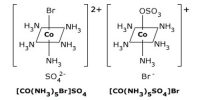
The coordination sphere is the metal ion and its coordinating ligands but not any uncoordinated counter-ions. It refers to the central metal ion and the ligands attached to it. In a formula of coordination compound, it is enclosed within the square brackets, ex. [Co(NH3)6]Cl. In this, the Co is central metal ion and NH3 is a ligand.
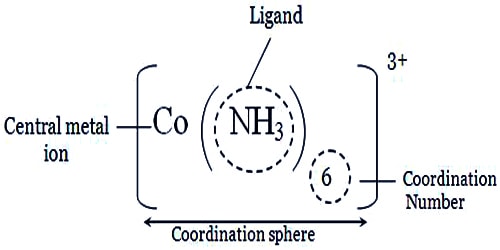
So, the coordination sphere is the region around a central atom or ion where linkage to ligands can occur to produce a complex. Coordination compounds are molecules that possess a metal center that is bound to ligands (atoms, ions, or molecules that donate electrons to the metal). These complexes can be neutral or charged. When the complex is charged, it is stabilized by neighboring counter-ions.
The coordination sphere is the collection of components of a coordination compound which includes the central atom and the ligands surrounding this central atom given along with the net electrical charge of the compound. Coordination chemistry emerged from the work of Alfred Werner, a Swiss chemist who examined different compounds composed of cobalt(III) chloride and ammonia. Upon the addition of hydrochloric acid, Werner observed that ammonia could not be completely removed. He then proposed that the ammonia must be bound more tightly to the central cobalt ion. The central metal atom is most of the time a positively charged component (a cation). Some ligands are neutrally charged (and contain lone electron pairs that can be donated) whereas other ligands are negatively charged (anions). Therefore, the net charge of the coordination compound is determined by both the central metal ion and the charge of ligands.