Conditions and Characteristics for adiabatic change
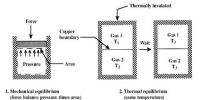
The process in which a system neither receives heat nor rejects heat is called adiabatic process. The change in which no heat is supplied from outside or heat is not ejected from the gas, but the change of pressure and volume takes place, is called adiabatic change.
Conditions for adiabatic change
The following conditions are needed to be fulfilled for adiabatic changes:
(a) The gas is to be kept in a bad-conducting container.
(b) Thermal capacity at the surroundings should be low.
(c) Change of pressure of the gas must be made very rapidly so that there is no chance of heat exchange with the surroundings.
Characteristics of adiabatic changes:
- Change of pressure and volume of a gas by keeping the total amount of gas constant is called adiabatic change.
- In this change temperature changes.
- It is a very fast process.
- In this change, the container should be a bad conductor.
- The thermal capacity of the surroundings should be low.
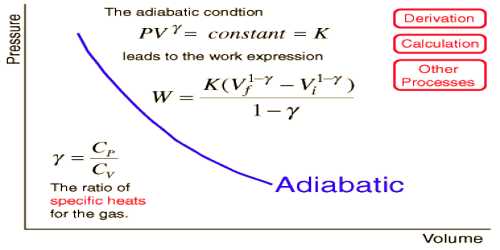
- For an ideal gas, the equation for adiabatic change is PVϒ = constant.
- Adiabatic curve is steeper than the isothermal curve.