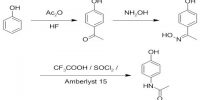
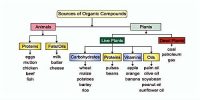
The energy required to remove the most loosely bound electron from an isolated atom in the gaseous state in known as Ionization Energy.
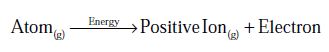 The ionization energy of an atom depends on the following factors (i) size of the atom (ii) charge on the nucleus (iii) screening effect of inner electrons (iv) penetration effect of electrons (v) effect of half-filled and completely filled sub-levels.
The ionization energy of an atom depends on the following factors (i) size of the atom (ii) charge on the nucleus (iii) screening effect of inner electrons (iv) penetration effect of electrons (v) effect of half-filled and completely filled sub-levels.
In a period, the value of ionization potential increases from left to right with breaks where the atoms have somewhat stable configurations. In a group, the ionization potential decreases from top to bottom.