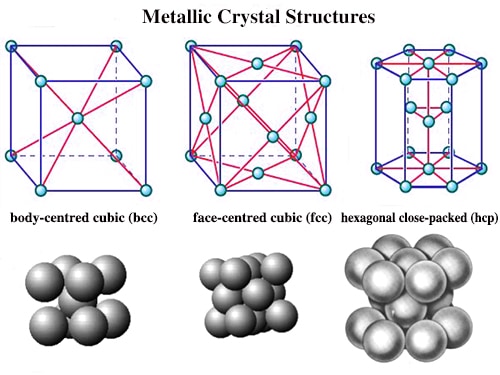
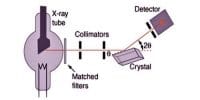
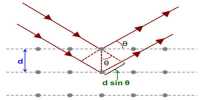
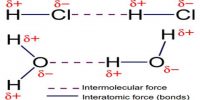
The metallic crystal consists of an assemblage of positive ions immersed in a sea of mobile electrons. Thus, each electron belongs to a number of positive ions and each positive ion belong to a number of electrons. The force that binds a metal ion to a number of electrons within its sphere of influence is known as metallic bond. This force of attraction is strong and is thus responsible for a compact solid structure of metals.