Properties of Copper:
Physical properties:
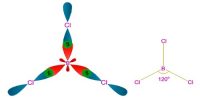
Copper is a reddish brown metal, with high lustre, high density and high melting point 1356°C.
Chemical Properties:
(i) Action of air and moisture
Copper gets covered with a green layer of basic copper carbonate, in the presence of CO2 and moisture
- 2Cu + O2 + CO2 + H2O → Cu(OH)2 . CuCO3 [(Green) Copper Carbonate]
ii) Action of Heat
Copper when heated to redness (below 1370K) in the presence of oxygen or air, first it gets converted to black cupric oxide and further heating to above 1370K, it gets converted into red cuprous oxide.
- 2Cu + O2 ⎯ Below 1370K→ 2CuO
- 4Cu + O2 ⎯ Above 1370 K→ 2Cu2O