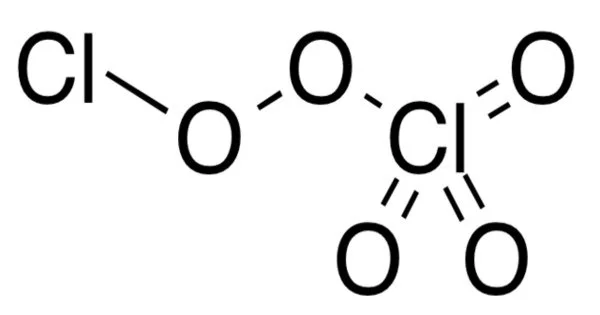
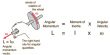
Dichlorine pentoxide, also known as Cl2O5, is a yellowish crystalline solid. Each chlorine atom is bonded to two oxygen atoms, resembling a chlorine molecule with two extra oxygen atoms attached to each chlorine. Dichlorine pentoxide has the molecular formula Cl2O5.
Dichlorine pentoxide is a fictitious chlorine oxide with the formula Cl2O5. Although the most stable configuration of dichlorine pentoxide is unknown, theory predicts that the perchloryl/chloride peroxide structure will be the most stable among various isomers, such as chloric acid anhydride or chlorous acid/perchloric acid mixed anhydride.
Properties:
- Physical State: It is a yellowish crystalline solid at room temperature.
- Odor: It has a pungent and suffocating odor.
- Melting Point: It has a melting point of around 85°C (185°F). At this temperature, it can decompose, so it is often handled at lower temperatures.
- Density: The density is approximately 2.32 g/cm³.
- Solubility: It is soluble in a variety of solvents, including chlorinated solvents, sulfuric acid, and nitric acid. However, it reacts violently with water, releasing chlorine gas and forming chloric acid.
Reactivity
Dichlorine pentoxide is a highly reactive compound. It is a powerful oxidizing agent and can react violently with various substances, such as water, organic compounds, reducing agents, and even some metals.
This compound is highly reactive and has the potential to be a powerful oxidizing agent. When it comes into contact with water, it reacts violently, releasing chlorine gas and forming chloric acid. The following equation can be used to represent the reaction with water:
Cl2O5 + H2O → 2HClO3
Application
Dichlorine pentoxide is primarily used as an intermediate compound in the synthesis of other chlorine compounds and as a powerful oxidizing agent in certain chemical reactions. However, due to its reactivity and potentially hazardous nature, it is not commonly encountered or used in everyday applications.
Hazardous
Due to its reactivity and potential hazards, dichlorine pentoxide should be handled with extreme caution. It is classified as a dangerous substance, and proper safety protocols, such as wearing protective equipment, should be followed when working with it.
It’s important to note that working with dichlorine pentoxide requires proper safety precautions and handling by trained professionals due to its corrosive and reactive nature.