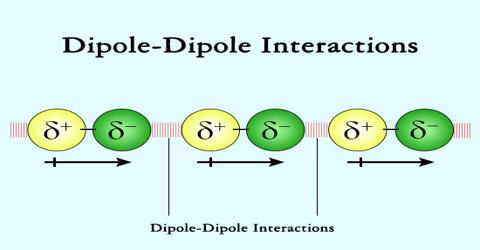
Dipole — Dipole interactions:
Dipole-dipole interactions were first described by Willem Hendrick Keesom in 1921.
These are the forces that occur between two polar molecules with permanent dipole moments. Dipole-dipole attractions are electrostatic in nature like the ionic bonds, but are weaker because only partial charges are involved. An example of this type of interactions can be seen in the hydrogen chloride molecules. In a Hydrochloric acid (HCl) molecule there is large difference in electro negativity between H atom and Cl atom (Cl is much more electronegative than H) and the electron pair between these two atoms in HCI molecule is attracted more strongly by Cl atom than by the H atom. This unsymmetrical (unequal) distribution of the electron pair between the combining atoms give rise to partial positive, charge (δ+) on the H atom and partial negative charge (δ-) on the Cl atom. As a result a dipole is formed, and the molecule is called polar. When two polar Hydrochloric acid molecules are close together a structure similar to the one shown below is focused.
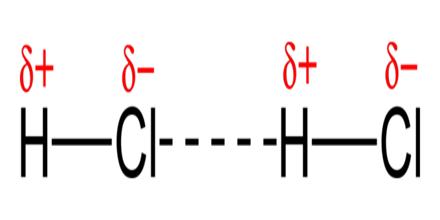
In a polyatomic molecule if the bond polarities do not cancel each other then the molecule has an overall polarity. This can happen because the symmetry in shape of the molecule. Examples are H2O and BF3 molecules. Water is polar, but BF3 is non-polar as shown below:
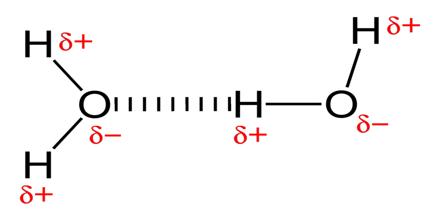
Figure: Shapes of H2O molecules
As can be seen, the bond polarities in BF3 cancel each other, because of the symmetrical planar shape of the molecule. In water, however, O – H bond polarities do not cancel as the molecule has a bent shape.