The enthalpy of a molecule, H, changes by dH when the temperature, T, changes by dT and the change is commonly given as a polynomial equation
dH = (a + bT + -cT2) dT
Calculate the change in the enthalpy, dH, of benzene (below Fig) when it is heated from 298 K to 398 K by integrating the polynomial given the following constants for benzene.
a = – 1.70 J mo1-1 K-1
b = 3.25 x 10-1 J mol-1 K-2
c = 1.11 x 10-4 J mo1-1 K-3
So,
Integrating from T1 to T2.
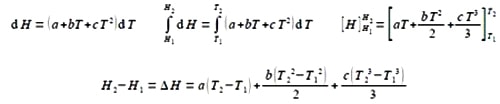
For clarity of presentation, below I have split the evaluation of the integrated equation over two lines
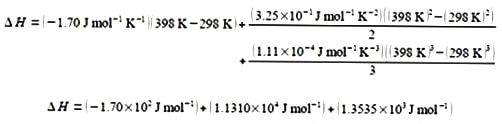
Δ H = 1.249 x 104 J mol-1
Δ H = 12.49 kJ mol-1
As a final step the enthalpy change of the benzene upon heating has been quoted in the conventional units of kJ mol-1.












