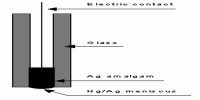
A large Kc indicates large concentrations of products at equilibrium as follows:
- Kc > 1000 means that the reaction goes to completion.
A small Kc indicates large concentrations of unreacted reactants at equilibrium:
- Kc < 0.001 means that the reaction does not happen.
The equilibrium law only applies to systems at equilibrium. The equilibrium constant, Kc, is the value obtained for the equilibrium-constant expression when equilibrium concentrations are substituted.
The numerical value of Kc is unaffected by changes in the concentrations of reactants or products. Kc is constant at constant temperature; however if the temperature changes, the value for the equilibrium constant will change.