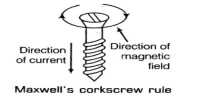
The passage of an electric current through a liquid causes chemical changes and this process is called electrolysis. The conduction is possible, only in liquids wherein charged ions can be dissociated in opposite directions (Figure).
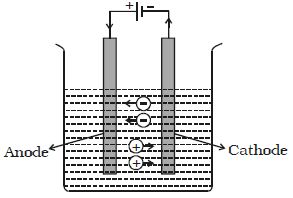
Such liquids are called electrolytes. The plates through which current enters and leaves an electrolyte are known as electrodes. The electrode towards which positive ions travel is called the cathode and the other, towards which negative ions travel is called anode. The positive ions are called cations and are mostly formed from metals or hydrogen. The negative ions are called anions.