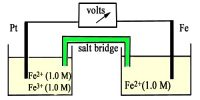
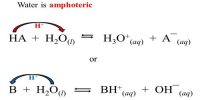
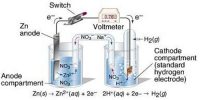
In the electrolysis of copper chloride, what mass of copper would be produced by a current of 1000 A running for 1 hour? First it is necessary to calculate the total charge passed using Faraday Calculations:
Q =I x t
Q = 1000 (A) x 60 x 60 (s) = 3,600,000 C
The reaction which involves copper is Cu2+ (aq) + 2e– → Cu (s)
So a charge equal to 2 x 96,500 C would be needed to produce one mole of copper. The number of moles of copper is equal to the total charge (in C) divided by the charge needed to produce one mol of copper as follows:
Moles Cu = 3,600,000 / (2 x 96,500)
Moles Cu = 18.65 mol
The mass of copper is equal to the moles of copper times the RAM of copper as follows:
Mass Cu = Moles Cu x RAM Cu
Mass Cu = 18.65 x 63.5 = 1,184 g
Example: When an aqueous solution of potassium iodide is electrolysed using platinum electrodes, the half-reactions are:
2I– (aq) → I2 (aq) + 2e–
2H2O (I) + 2e– → H2 (g) + 2OH– (aq)
How many grams of iodine are produced when a current of 8.52 mA flows through the cell for 10 min?
Ans: When the current flows for 6.00 x 102 s (i.e. 10.0 min),
the amount of charge is equal to:
(8.52 x 10-3 A) x (6.00 x 102 s) = 5.11 C
Two moles of electrons are equivalent to one mole of I2. This gives us the following calculation: Mass = 5.11 C x (1 mol e– / 96,500) x (1 mol I2 / 2 mol e–) x (254g I2 / 1 mol I2) Mass I2 = 6.73 x 10-3 g