Ionic Compounds: Ionic compounds are crystalline solids. A crystal has a regular shape with flat sides (“faces”), and consistent angles between the faces. This is because the atoms are arranged in an orderly repeating pattern. Sodium chloride is taken as a typical ionic compound. Compounds like this consist of a giant (endlessly repeating) lattice of ions. So sodium chloride (and any other ionic compound) is described as having a giant ionic structure.
Structure of Ionic Compounds: Ionic compounds contain positive and negative ions. The ions in the crystal are arranged in a ‘giant’, 3- dimensional structure called an ionic lattice.
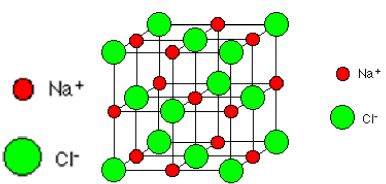
If you look at the diagram carefully, you will see that the sodium ions and chloride ions alternate with each other in each of the three dimensions. The lattice is held together by electrostatic attraction between the cations and anions. Remember that ionic structures are only found in compounds (i.e. between atoms of different elements).